Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0652) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Eszopiclone
|
|||||
| Synonyms |
Esopiclone; Estorra; Eszopiclone; Eszopiclone [USAN:INN]; Lunesta; SEP-190; SEP-225441; UZX80K71OE; ( )-Zopiclone; (+)-Zopiclone; (5S)-6-(5-CHLOROPYRID-2-YL)-5-(4-METHYLPIPERAZIN-1-YL)CARBONYLOXY-7-OXO-6,7-DIHYDRO-5H-PYRROLO[3,4-B]PYRAZINE; (5S)-6-(5-chloropyridin-2-yl)-7-oxo-5H,6H,7H-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate; (S)-Zopiclone; 138729-47-2; CHEBI:53760; HSDB 7472; UNII-UZX80K71OE; [(7S)-6-(5-chloropyridin-2-yl)-5-oxo-7H-pyrrolo[3,4-b]pyrazin-7-yl] 4-methylpiperazine-1-carboxylate
|
|||||
| Indication | Insomnia [ICD11: 7A00] | Approved | [1] | |||
| Structure |
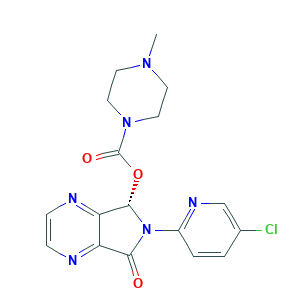 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 388.8 | Topological Polar Surface Area | 91.8 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 7 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

