| General Information of Drug (ID:
DR0708) |
| Drug Name |
Flunarizine
|
| Synonyms |
Flunarizin; Flunarizina; Flunarizina [INN-Spanish]; Flunarizine [INN:BAN]; Flunarizinum; Flunarizinum [INN-Latin]; R7PLA2DM0J; Sibelium; flunarizine; (E)-1-(Bis(4-fluorophenyl)methyl)-4-(3-phenyl-2-propenyl)piperazine; (E)-1-[Bis-(p-fluorophenyl)methyl]-4-cinnamylpiperazine; 1-(Bis(4-fluorophenyl)methyl)-4-cinnamylpiperazine; 1-[bis(4-fluorophenyl)methyl]-4-(3-phenylprop-2-en-1-yl)piperazine; 52468-60-7; CHEMBL30008; EINECS 257-937-5; Piperazine, 1-(bis(4-fluorophenyl)methyl)-4-(3-phenyl-2-propenyl)-, (E)-; UNII-R7PLA2DM0J
|
| Indication |
Schizophrenia
[ICD11: 6A20]
|
Phase 4
|
[1]
|
| Structure |
|
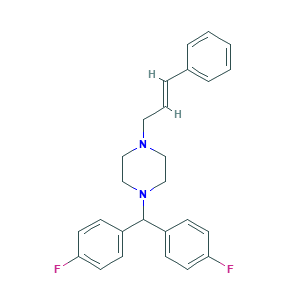 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
404.5 |
Topological Polar Surface Area |
6.5 |
| Heavy Atom Count |
30 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 941361
- PubChem SID
-
7979238
; 9208288
; 10525243
; 14879384
; 26751607
; 26751608
; 44891609
; 46507129
; 47216516
; 47216517
; 47439976
; 47515052
; 47515053
; 47515054
; 47515055
; 47588728
; 47588729
; 47736197
; 48184716
; 49698432
; 50011452
; 50104264
; 50104265
; 56365814
; 57408889
; 80028030
; 81093273
; 85787717
; 85788409
; 85788604
; 90341204
; 92308837
; 92309757
; 92711460
; 96024666
; 99300671
; 99302189
; 103050123
; 103096316
; 103153578
; 103199419
; 103928970
; 104179181
; 110096637
; 117509719
; 124526360
; 124749784
; 124883264
; 124883265
; 124883266
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05CEU
- Formula
- C26H26F2N2
- Canonical SMILES
- C1CN(CCN1CC=CC2=CC=CC=C2)C(C3=CC=C(C=C3)F)C4=CC=C(C=C4)F
- InChI
- 1S/C26H26F2N2/c27-24-12-8-22(9-13-24)26(23-10-14-25(28)15-11-23)30-19-17-29(18-20-30)16-4-7-21-5-2-1-3-6-21/h1-15,26H,16-20H2/b7-4+
- InChIKey
- SMANXXCATUTDDT-QPJJXVBHSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


