| General Information of Drug (ID:
DR0809) |
| Drug Name |
VA-10872
|
| Synonyms |
Barbidorm; Citodon; Citopan; Cyclonal; Cyclopan; Hexobarbital; Dorico; Enhexymal; Enhexymalum; Esobarbitale; Esobarbitale [DCIT]; Esobarbitale [Italian]; Evipal; Evipan; Hexabarbital; Hexanastab oral; Hexenal (barbiturate); Hexobarbital (VAN); Hexobarbitalum; Hexobarbitalum [INN-Latin]; Hexobarbitone; Hexobarbitonum; Methexenyl; Methylhexabarbital; Methylhexabital; Narcosan; Noctivane; Sodium hexobarbital; Sombucaps; Sombulex; Somnalert; 5-(1-CYCLOHEXEN-1-YL)-1,5-DIMETHYLBARBITURIC ACID; 56-29-1
|
| Indication |
Anaesthesia
[ICD11: 8E22]
|
Preclinical
|
[1]
|
| Structure |
|
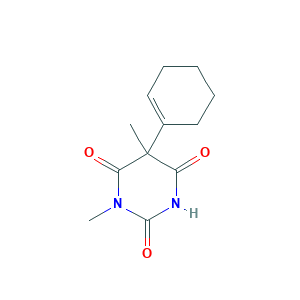 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
236.27 |
Topological Polar Surface Area |
66.5 |
| Heavy Atom Count |
17 |
Rotatable Bond Count |
1 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 3608
- PubChem SID
-
13888
; 114792
; 4911070
; 7848134
; 8147019
; 8152276
; 10527424
; 15415683
; 24895418
; 26754503
; 29215369
; 29222734
; 46508777
; 47194291
; 47959959
; 48416081
; 49873997
; 57309846
; 57321894
; 57654225
; 79698506
; 92714691
; 103165870
; 104304001
; 104903183
; 117838626
; 118856010
; 124813353
; 124892192
; 125309499
; 125488787
; 126623945
; 129440427
; 134337977
; 134972602
; 135194968
; 135300355
; 137101291
; 139408774
; 144205214
; 144205215
; 144236491
; 160964645
; 163050404
; 164815041
; 170250749
; 170465860
; 176267018
; 179225803
; 179296095
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00ETS
- Formula
- C12H16N2O3
- Canonical SMILES
- CC1(C(=O)NC(=O)N(C1=O)C)C2=CCCCC2
- InChI
- 1S/C12H16N2O3/c1-12(8-6-4-3-5-7-8)9(15)13-11(17)14(2)10(12)16/h6H,3-5,7H2,1-2H3,(H,13,15,17)
- InChIKey
- UYXAWHWODHRRMR-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


