| Synonyms |
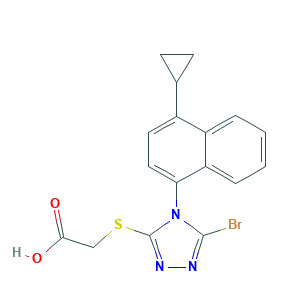
Lesinured [USAN]; AK323774; C17H14BrN3O2S; UNII-09ERP08I3W; LESINURAD; RDEA 594; RDEA-594; RDEA594; Zurampic; 09ERP08I3W; 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetic acid; 2-(5-Bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio)acetic acid; 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-1,2,4-triazol-3-yl]sulfanyl]acetic acid; 2-[[5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl]thio]acetic acid; 878672-00-5
|
| Cross-matching ID |
- PubChem CID
- 53465279
- PubChem SID
-
135267370
; 135626658
; 141682643
; 160698094
; 163620844
; 163686172
; 186022493
; 198964591
; 223366008
; 224297829
; 227115271
; 243322229
; 251963128
; 252216185
; 252440600
; 252479355
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C3SW
- Formula
- C17H14BrN3O2S
- Canonical SMILES
- C1CC1C2=CC=C(C3=CC=CC=C23)N4C(=NN=C4Br)SCC(=O)O
- InChI
- 1S/C17H14BrN3O2S/c18-16-19-20-17(24-9-15(22)23)21(16)14-8-7-11(10-5-6-10)12-3-1-2-4-13(12)14/h1-4,7-8,10H,5-6,9H2,(H,22,23)
- InChIKey
- FGQFOYHRJSUHMR-UHFFFAOYSA-N
|