| Synonyms |
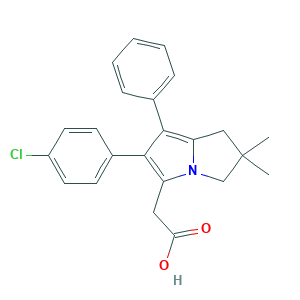
Licofelone; Licofelone [INN]; ML 3000; ML-3000; P5T6BYS22Y; 156897-06-2; 2,3-Dihydro-6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-1H-pyrrolizine-5-acetic acid; 2-[2-(4-chlorophenyl)-6,6-dimethyl-1-phenyl-5,7-dihydropyrrolizin-3-yl]acetic acid; 6-(4-Chlorophenyl)-2,3-dihydro-2,2-dimethyl-7-phenyl-1H-pyrrolizine-5-acetic acid; BRN 6823674; C23H22ClNO2; CHEMBL300982; UNII-P5T6BYS22Y; [6-(4-CHLOROPHENYL)-2,2-DIMETHYL-7-PHENYL-2,3-DIHYDRO-1H-PYRROLIZIN-5-YL]ACETIC ACID
|
| Cross-matching ID |
- PubChem CID
- 133021
- PubChem SID
-
6436380
; 7888616
; 10243273
; 12014886
; 14829304
; 29311030
; 50040041
; 50700417
; 53788587
; 57344354
; 103243188
; 103945934
; 104378798
; 125333743
; 126645607
; 126648934
; 129342134
; 131300446
; 134338958
; 135088300
; 135692433
; 137116560
; 137275875
; 142116170
; 144072480
; 162172234
; 162476140
; 163884604
; 164228207
; 164761582
; 175426859
; 184826912
; 196105884
; 198972330
; 204370171
; 223392829
; 223705238
; 223909705
; 226588726
; 251882412
; 252215695
; 252450609
- CAS Number
-
- TTD Drug ID
- D0N1SU
- Formula
- C23H22ClNO2
- Canonical SMILES
- CC1(CC2=C(C(=C(N2C1)CC(=O)O)C3=CC=C(C=C3)Cl)C4=CC=CC=C4)C
- InChI
- 1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27)
- InChIKey
- UAWXGRJVZSAUSZ-UHFFFAOYSA-N
|