| General Information of Drug (ID:
DR0123) |
| Drug Name |
Apomorphine
|
| Synonyms |
Apokyn; Apomorfin; Apomorphin; Apomorphine (BAN); Apomorphine [BAN]; Apomorphine hydrochloride; Apomorphinium chloride hemihydrate; Apormorphine; Ixense; L-Apomorphine; R-(-)-Apomorphine; Uprima; Uprima (TN); VR-040; VR-400; apomorphine; (-)-10,11-Dihydroxyaporphine; 58-00-4; 6a-beta-Aporphine-10,11-diol; 6abeta-Aporphine-10,11-diol; Apomorphine hydrochloride hemihydrate; C17H17NO2; CHEBI:48538; CHEMBL53; DSSTox_CID_2614; EINECS 200-360-0; HSDB 3289; N21FAR7B4S; NCGC00025349-02; UNII-N21FAR7B4S; VR004
|
| Indication |
Parkinsonism
[ICD11: 8A00]
|
Approved
|
[1]
|
| Structure |
|
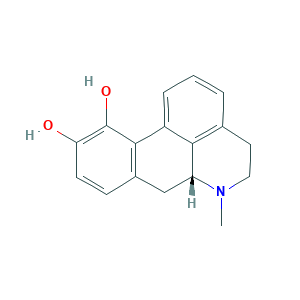 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
267.32 |
Topological Polar Surface Area |
43.7 |
| Heavy Atom Count |
20 |
Rotatable Bond Count |
0 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
3 |
| Cross-matching ID |
- PubChem CID
- 6005
- PubChem SID
-
7978719
; 8153735
; 11114270
; 11466129
; 11467249
; 11485757
; 14823961
; 14848260
; 24262970
; 26752261
; 29225019
; 46508653
; 47216673
; 47365074
; 47365075
; 47662161
; 47662162
; 47662163
; 48110346
; 48184887
; 48259117
; 48415574
; 49658769
; 49698343
; 49871434
; 50322702
; 51091791
; 57323108
; 85788349
; 85856298
; 92308726
; 92729815
; 93622853
; 103167211
; 103916308
; 104310845
; 124887059
; 124887060
; 126522630
; 129497604
; 134337595
; 134971357
; 135304977
; 135649950
; 137001467
; 142333205
; 144204518
; 160964058
; 163667986
; 164788060
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0H6QU
- Formula
- C17H17NO2
- Canonical SMILES
- CN1CCC2=C3C1CC4=C(C3=CC=C2)C(=C(C=C4)O)O
- InChI
- 1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1
- InChIKey
- VMWNQDUVQKEIOC-CYBMUJFWSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


