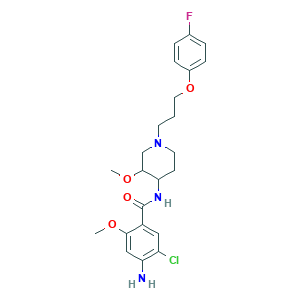
| Cross-matching ID |
- PubChem CID
- 2769
- PubChem SID
-
9127
; 4816531
; 7847340
; 8151787
; 10321734
; 11466458
; 11467578
; 11486095
; 14882747
; 29221924
; 48403970
; 48415785
; 49875392
; 50113158
; 51031281
; 53786866
; 57321449
; 80953524
; 85209640
; 92125386
; 92711730
; 99418016
; 103914221
; 103919261
; 104301556
; 123090165
; 125358025
; 125663585
; 126630574
; 126657130
; 126669689
; 127715812
; 131993450
; 134337537
; 135014802
; 135650104
; 137101280
; 142378524
; 144204250
; 160963949
; 162357904
; 162771742
; 164807926
; 165700416
; 170465200
; 175611161
; 178125987
; 179148462
; 184551724
; 196112081
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0BD8D
- Formula
- C23H29ClFN3O4
- Canonical SMILES
- COC1CN(CCC1NC(=O)C2=CC(=C(C=C2OC)N)Cl)CCCOC3=CC=C(C=C3)F
- InChI
- 1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29)
- InChIKey
- DCSUBABJRXZOMT-UHFFFAOYSA-N
|