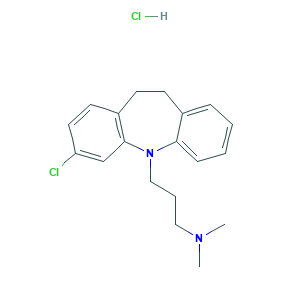
| Synonyms |
Chlorimipramine hydrochloride; Chloroimipramine monohydrochloride; Clomipramine (hydrochloride); Clomipramine HCL; Clomipramine Monohydrochloride; Clomipramine hydrochloride; Chlorimipramine; Clomipramine; Clomipramina; Clomipramina [INN-Spanish]; Clomipramine [INN:BAN]; Clomipraminum; Clomipraminum [INN-Latin]; Hydiphen; Monochlorimipramine; NSC 169865; UNII-NUV44L116D; 3-(3-chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine; 3-Chloro-5-(3-(dimethylamino)propyl)-10,11-dihydro-5H-dibenz(b,f)azepine; 3-Chloroimipramine; 303-49-1; 5H-Dibenz[b,f]azepine-5-propanamine, 3-chloro-10,11-dihydro-N,N-dimethyl-; Anafranil (free base); Anafranil base; EINECS 241-344-3; I615; MFCD00069234; MLS000028511; SMR000058295; UNII-2LXW0L6GWJ; 17321-77-6; 2LXW0L6GWJ; 3-(3-chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine hydrochloride; 3-Chloroimipramine hydrochloride; Anafranil; Anaphranil; C19H23ClN2.HCl; CHEBI:3755; CPD000058295
|
| Cross-matching ID |
- PubChem CID
- 68539
- PubChem SID
-
855785
; 3146444
; 7847876
; 8150146
; 8192195
; 10321173
; 11528647
; 14925416
; 17404870
; 24278087
; 24714730
; 25624751
; 26514064
; 26543492
; 26613159
; 26680358
; 26747483
; 43125096
; 50064402
; 50106071
; 50122894
; 50122895
; 53777413
; 56311677
; 56422907
; 57316916
; 79827483
; 85084917
; 85149187
; 85174244
; 88565714
; 92125084
; 92303620
; 103770693
; 103819575
; 103913653
; 104170176
; 104342415
; 117447143
; 124637328
; 124891638
; 124891639
; 125312387
; 125342946
; 126617326
; 126645822
; 126664318
; 135027196
; 135610155
; 135697887
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D05AAW
- Formula
- C19H24Cl2N2
- Canonical SMILES
- CN(C)CCCN1C2=CC=CC=C2CCC3=C1C=C(C=C3)Cl.Cl
- InChI
- 1S/C19H23ClN2.ClH/c1-21(2)12-5-13-22-18-7-4-3-6-15(18)8-9-16-10-11-17(20)14-19(16)22;/h3-4,6-7,10-11,14H,5,8-9,12-13H2,1-2H3;1H
- InChIKey
- WIMWMKZEIBHDTH-UHFFFAOYSA-N
|