Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0363) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Clozapine
|
|||||
| Synonyms |
Clorazil; Clozapin; Clozapina; Clozapina [INN-Spanish]; Clozapinum; Clozapinum [INN-Latin]; Fazaclo; Fazaclo ODT; HF 1854; Asaleptin; CLOZARIL; HF-1854; LX 100-129; Leponex; Lepotex; clozapine; 5786-21-0; 5H-Dibenzo[b,e][1,4]diazepine, 8-chloro-11-(4-methyl-1-piperazinyl)-; 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo(b,e)(1,4)diazepine; 8-Chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine; 8-chloro-11-(4-methylpiperazin-1-yl)-5H-dibenzo[b,e][1,4]diazepine; CCRIS 9171; HSDB 6478; Iprox; UNII-J60AR2IKIC; W-801
|
|||||
| Indication | Schizophrenia [ICD11: 6A20] | Approved | [1] | |||
| Structure |
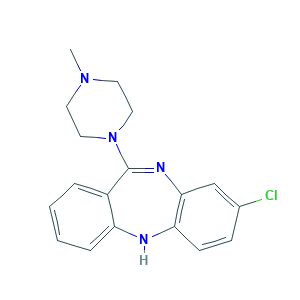 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 326.8 | Topological Polar Surface Area | 30.9 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

