| Synonyms |
Fosphenytoin (disodium); Fosphenytoin (sodium); Fosphenytoin disodium; Fosfenitoina; Fosfenitoina [INN-Spanish]; Fosphenytoin; Fosphenytoin (INN); Fosphenytoin [INN:BAN]; Fosphenytoine; Fosphenytoine [INN-French]; Fosphenytoinum; Fosphenytoinum [INN-Latin]; Prodilantin; (2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl dihydrogen phosphate; (3-Phosphoryloxymethyl)phenytoin; 2,4-Imidazolidinedione, 5,5-diphenyl-3-((phosphonooxy)methyl)-; 2,4-Imidazolidinedione, 5,5-diphenyl-3-((phosphonooxy)methyl)-, (SP-4-2)-; 93390-81-9; B4SF212641; CHEBI:5165; HMPDP; UNII-B4SF212641; Fosphenytoin disodium salt; Fosphenytoin sodium; Fosphenytoin sodium [USAN]; Fosphenytoin_Sodium; Fostoin; Pro-Epanutin; fospenytoin; 2,4-Imidazolidinedione, 5,5-diphenyl-3-((phosphonooxy)methyl)-, disodium salt; 3-(Hydroxymethyl)-5,5-diphenylhydantoin, disodium phosphate (ester); 7VLR55452Z; 92134-98-0; ACC 9653; ACC 9653-010; ACC-9653-010; CI 982; CI-982; UNII-7VLR55452Z; disodium;(2,5-dioxo-4,4-diphenylimidazolidin-1-yl)methyl phosphate
|
| Cross-matching ID |
- PubChem CID
- 56338
- CAS Number
-
- TTD Drug ID
- D0J5YC
- Formula
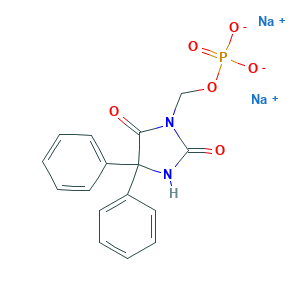
- C16H13N2Na2O6P
- Canonical SMILES
- C1=CC=C(C=C1)C2(C(=O)N(C(=O)N2)COP(=O)([O-])[O-])C3=CC=CC=C3.[Na+].[Na+]
- InChI
- 1S/C16H15N2O6P.2Na/c19-14-16(12-7-3-1-4-8-12,13-9-5-2-6-10-13)17-15(20)18(14)11-24-25(21,22)23;;/h1-10H,11H2,(H,17,20)(H2,21,22,23);;/q;2*+1/p-2
- InChIKey
- GQPXYJNXTAFDLT-UHFFFAOYSA-L
|