| General Information of Drug (ID:
DR0801) |
| Drug Name |
Haloperidol decanoate
|
| Prodrug Info |
Haloperidol decanoate is the prodrug of Reduced haloperidol
|
| Synonyms |
Haloperidolum; Halopidol; Halopoidol; Halosten; Keselan; Lealgin compositum; Linton; McN-JR-1625; Mixidol; Pekuces; Peluces; Pernox; Serenace; Serenase; Serenelfi; Sernas; Sernel; Sigaperidol; Ulcolind; Uliolind; Vesalium; haloperidol; 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one; 52-86-8; Aldo; Dozic; Halol; Aloperidin; Aloperidol; Aloperidolo; Aloperidolo [DCIT]; Aloperidolo [Italian]; Aloperidon; Bioperidolo; Brotopon; Einalon S; Eukystol; Fortunan; Galoperidol; Haldol; Haldol Solutab; Halidol; Halojust; Halopal; Depot haloperidol; HALOPERIDOL DECANOATE; Haldol decanoas; Haldol decanoate; Halomonth; Haloperidol depot; KD 136; KD-136; Neoperidole; R 13,672; R 13672; R-13672; 4-(4-Chlorophenyl)-1-(4-(4-fluorophenyl)-4-oxobutyl)-4-piperidyl decanoate; 74050-97-8; AC20PJ4101; C31H41ClFNO3; Decanoic acid, 4-(4-chlorophenyl)-1-(4-(4-fluorophenyl)-4-oxobutyl)-4-piperidinyl ester; Decanoic acid, ester with 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone; EINECS 277-679-7; KD 16; UNII-AC20PJ4101
|
| Indication |
Agitation/aggression
[ICD11: 6D86]
|
Approved
|
[1]
|
| Structure |
|
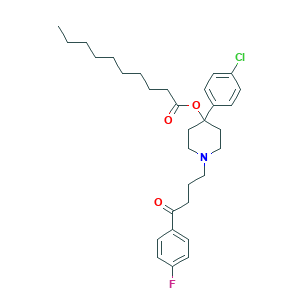 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
530.1 |
Topological Polar Surface Area |
46.6 |
| Heavy Atom Count |
37 |
Rotatable Bond Count |
16 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 52919
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0D1AL
- Formula
- C31H41ClFNO3
- Canonical SMILES
- CCCCCCCCCC(=O)OC1(CCN(CC1)CCCC(=O)C2=CC=C(C=C2)F)C3=CC=C(C=C3)Cl
- InChI
- 1S/C31H41ClFNO3/c1-2-3-4-5-6-7-8-11-30(36)37-31(26-14-16-27(32)17-15-26)20-23-34(24-21-31)22-9-10-29(35)25-12-18-28(33)19-13-25/h12-19H,2-11,20-24H2,1H3
- InChIKey
- GUTXTARXLVFHDK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


