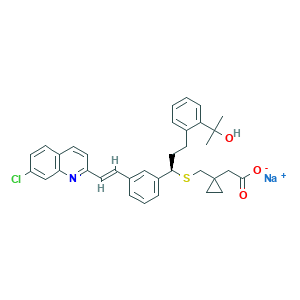
| Synonyms |
Montelukast (sodium); Montelukast sodium [USAN]; Montelukast sodium salt; Montelukast [INN:BAN]; Singular; montelukast; (R-(E))-1-(((1-(3-(2-(7-Chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid; 1-((((1R)-1-(3-((1E)-2-(7-Chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)cyclopropaneacetic acid; 158966-92-8; CHEBI:50730; CHEMBL787; UNII-MHM278SD3E; MHM278SD3E; U1O3J18SFL; 151767-02-1; CHEBI:6993; CPD000469188; Cyclopropaneacetic acid, 1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]-, sodium salt (1:1); DSSTox_CID_26450; DSSTox_GSID_46450; DSSTox_RID_81624; UNII-U1O3J18SFL; MK 476; MK-0476; MK-476; MONTELUKAST Na; MONTELUKAST SODIUM; Montair
|
| Cross-matching ID |
- PubChem CID
- 23663996
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00QET
- Formula
- C35H35ClNNaO3S
- Canonical SMILES
- CC(C)(C1=CC=CC=C1CCC(C2=CC=CC(=C2)C=CC3=NC4=C(C=CC(=C4)Cl)C=C3)SCC5(CC5)CC(=O)[O-])O.[Na+]
- InChI
- 1S/C35H36ClNO3S.Na/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29;/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39);/q;+1/p-1/b15-10+;/t32-;/m1./s1
- InChIKey
- LBFBRXGCXUHRJY-HKHDRNBDSA-M
|