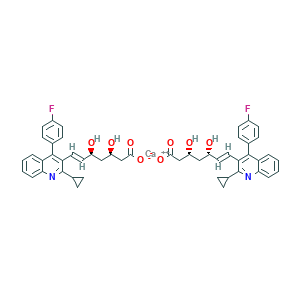
| Synonyms |
Pitavastatin; Pitavastatin [INN]; Zypitamag; ( )-(3R,5S,6E)-7-(2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoic acid; (3R,5S,6E)-7-(2-Cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoic acid; (3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoic acid; C25H24FNO4; CHEBI:32020; UNII-M5681Q5F9P; Itavastatin; M5681Q5F9P; NK 104; Alipza; IYD54XEG3W; Itavastatin calcium; Livazo; Nisvastatin; Pitavastatin hemicalcium; 147526-32-7; 2C25H23FNO4.Ca; Bis((3R,5S,6E)-7-(2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoate), monocalcium salt; CHEBI:71258; Calcium (3R,5S,E)-7-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoate; UNII-IYD54XEG3W
|
| Cross-matching ID |
- PubChem CID
- 5282451
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0G1WL
- Formula
- C50H46CaF2N2O8
- Canonical SMILES
- C1CC1C2=NC3=CC=CC=C3C(=C2C=CC(CC(CC(=O)[O-])O)O)C4=CC=C(C=C4)F.C1CC1C2=NC3=CC=CC=C3C(=C2C=CC(CC(CC(=O)[O-])O)O)C4=CC=C(C=C4)F.[Ca+2]
- InChI
- 1S/C25H24FNO4.Ca/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31;/h1-4,7-12,16,18-19,28-29H,5-6,13-14H2,(H,30,31);/q;+2/b12-11+;/t18-,19-;/m1./s1
- InChIKey
- RHGYHLPFVJEAOC-FFNUKLMVSA-L
|