| General Information of Drug (ID:
DR5206) |
| Drug Name |
Selegiline
|
| Synonyms |
selegiline; L-Deprenalin; Emsam; (-)-selegiline; Selegilinum; Selegilina; Carbex; 14611-51-9; Selegilinum [INN-Latin]; Selegilina [INN-Spanish]; UNII-2K1V7GP655; l-E 250; CHEMBL972; CHEBI:9086; N-methyl-N-[(2R)-1-phenylpropan-2-yl]prop-2-yn-1-amine; 2K1V7GP655; (R)-(-)-N,alpha-Dimethyl-N-2-propinylphenethylamine; Benzeneethanamine, N,alpha-dimethyl-N-2-propynyl-, (R)-; selgene; (R)-(-)-N-Methyl-N-(1-phenyl-2-propyl)-2-propinylamin; Selegyline; Zalapar; Selegiline (transdermal, Parkinson's/depression); Zunrisa/Rezonic
|
| Indication |
Major depressive disorder
[ICD11: 6A70-6A7Z]
|
Approved
|
[1]
|
|
Chemotherapy-induced nausea
[ICD11:
ICD11: MD90]
|
Discontinued in Phase 3
|
[2]
|
|
Skin imperfections
[ICD11:
ICD11: EK71]
|
Patented
|
[3]
|
| Structure |
|
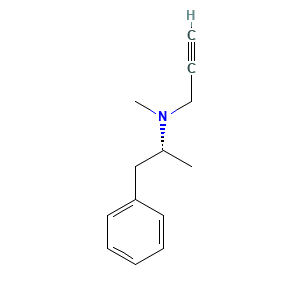 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
187.28 |
Topological Polar Surface Area |
3.2 |
| Heavy Atom Count |
14 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
1 |
| Cross-matching ID |
- PubChem CID
- 26757
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0S2UG
- Formula
- C13H17N
- Canonical SMILES
- C[C@H](CC1=CC=CC=C1)N(C)CC#C
- InChI
- InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1
- InChIKey
- MEZLKOACVSPNER-GFCCVEGCSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


