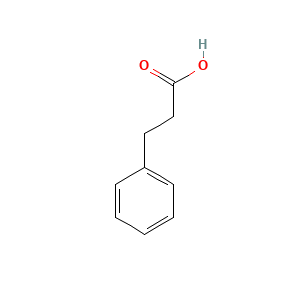
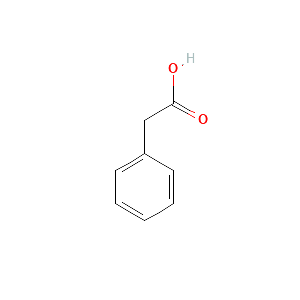
| References |
| 1 |
High gastrointestinal permeability and local metabolism of naringenin: influence of antibiotic treatment on absorption and metabolism
|
| 2 |
In vitro catabolism of 3',4'-dihydroxycinnamic acid by human colonic microbiota
|
| 3 |
Catabolism of citrus flavanones by the probiotics Bifidobacterium longum and Lactobacillus rhamnosus. Eur J Nutr. 2018 Feb;57(1):231-242.
|
| 4 |
Microbial Metabolism of Naringin and the Impact on Antioxidant Capacity
|
| 5 |
Biotransformation of two citrus flavanones by lactic acid bacteria in chemical defined medium
|
| 6 |
Ipratropium bromide: drug metabolism and pharmacokinetics
|
| 7 |
Ligand-induced conformational change in penicillin acylase
|
| 8 |
A breakthrough in enzyme technology to fight penicillin resistance-industrial application of penicillin amidase
|
| 9 |
Insights into the mechanisms of action of the MAO inhibitors phenelzine and tranylcypromine: a review J Psychiatry Neurosci. 1992 Nov;17(5):206-14.
|
| 10 |
DrugBank(Pharmacology-Metabolism):Sodium phenylbutyrate
|