Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0583) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Enzalutamide
|
|||||
| Synonyms |
Enzalutamide; Enzalutamide (MDV3100); MDV 3100; MDV-3100; MDV3100; MDV3100 (Enzalutamide); MDV3100 Enzalutamide; XTANDI; 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide; 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl}-2-fluoro-N-methylbenzamide; 915087-33-1; 93T0T9GKNU; CHEBI:68534; UNII-93T0T9GKNU
|
|||||
| Indication | Prostate cancer [ICD11: 2C82] | Approved | [1] | |||
| Structure |
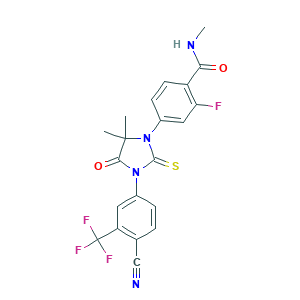 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 464.4 | Topological Polar Surface Area | 109 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Enzalutamide was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | DrugBank(Pharmacology-Metabolism):Enzalutamide | |||||
| 3 | Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016 Jan;68(1):168-241. | |||||
| 4 | [Enzalutamide in castration resistant prostate cancer.] | |||||
| 5 | Enzalutamide: an evidence-based review of its use in the treatment of prostate cancer. Core Evid. 2013;8:27-35. | |||||
| 6 | Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet. 2015 Oct;54(10):1057-69. | |||||
| 7 | Product Monograph of Xtandi. | |||||
| 8 | Absorption, Distribution, Metabolism, and Excretion of the Androgen Receptor Inhibitor Enzalutamide in Rats and Dogs | |||||
| 9 | Pharmacokinetic Aspects of the Two Novel Oral Drugs Used for Metastatic Castration-Resistant Prostate Cancer: Abiraterone Acetate and Enzalutamide | |||||
| 10 | Clinical Pharmacokinetic Studies of Enzalutamide | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

