| Synonyms |
Imatinib; Imatinib (STI571); Imatinib Methansulfonate; Imatinib [INN:BAN]; Imatinib free base; Imatinib(STI571); Gleevec; Glivec; Imatinib (mesylate); Imatinib Mesylate (STI571); Imatinib mesilate; Imatinib mesylate; Imatinib mesylate (USAN); Imatinib mesylate, 98%; Imatinib, methanesulfonate salt; ST-1571 Mesylate; imatinib methanesulfonate; imatinib monomesylate; 220127-57-1; 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-benzamide monomethanesulfonate; 8A1O1M485B; AK-44930; CGP-57148B; CHEBI:31690; HSDB 7142; NSC-716051; UNII-8A1O1M485B; STI571; 152459-95-5; 4-(4-METHYL-PIPERAZIN-1-YLMETHYL)-N-[4-METHYL-3-(4-PYRIDIN-3-YL-PYRIMIDIN-2-YLAMINO)-PHENYL]-BENZAMIDE; CCRIS 9076; CGP-57148; CHEMBL941; Cgp 57148; N-(4-Methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide; BKJ8M8G5HI; UNII-BKJ8M8G5HI
|
| Cross-matching ID |
- PubChem CID
- 123596
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0AZ3C
- Formula
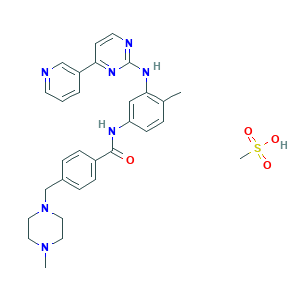
- C30H35N7O4S
- Canonical SMILES
- CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5.CS(=O)(=O)O
- InChI
- 1S/C29H31N7O.CH4O3S/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;1-5(2,3)4/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H3,(H,2,3,4)
- InChIKey
- YLMAHDNUQAMNNX-UHFFFAOYSA-N
|