Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1163) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Nintedanib esylate
|
|||||
| Synonyms |
Nintedanib Esilate; Nintedanib Esylate(BIBF-1120); Nintedanib Ethanesulfonate Salt; Nintedanib esylate; Nintedanib ethanesulfonate; SCHEMBL2278759; SCHEMBL753253; (3Z)-2,3-Dihydro-3-[[[4-[methyl[2-(4-methyl-1-piperazinyl)acetyl]amino]phenyl]amino]phenylmethylene]-2-oxo-1H-indole-6-carboxylic acid methyl ester ethanesulfonate; 42F62RTZ4G; 656247-18-6; AKOS025149444; BCP09963; BIBF 1120 (esylate); BIBF 1120 esylate; CHEBI:85170; CHEMBL3039504; DTXSID40215873; KS-00000LA8; MFCD26142360; NSC753000; UNII-42F62RTZ4G
|
|||||
| Indication | Idiopathic pulmonary fibrosis [ICD11: CB03] | Approved | [1] | |||
| Structure |
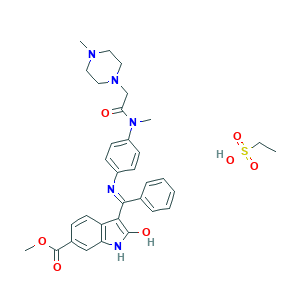 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 649.8 | Topological Polar Surface Area | 164 | ||
| Heavy Atom Count | 46 | Rotatable Bond Count | 9 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 10 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Nintedanib Esylate was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin Pharmacokinet. 2019 Sep;58(9):1131-1147. | |||||
| 3 | Metabolic pro?ling of tyrosine kinase inhibitor nintedanib using metabolomics J Pharm Biomed Anal. 2020 Feb 20;180:113045. doi: 10.1016/j.jpba.2019.113045. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

