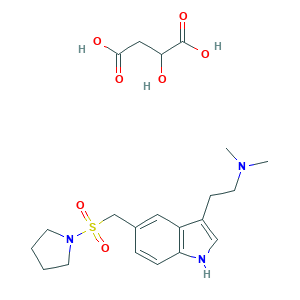
| Synonyms |
Almotriptan; Almotriptan (malate); Almotriptan malate; Almotriptan malate (USAN); Almotriptan malate [USAN]; Almotriptan maleate; CHEBI:53781; LAS 31416; LAS 31416 D,L-malate acid; PNU 180638E; PNU-180638E; Almogran; Almotriptan [USAN:INN:BAN]; Axert; CHEBI:520985; DSSTox_CID_24289; DSSTox_GSID_44289; DSSTox_RID_80142; LAS-31416; N,N-dimethyl-2-(5-((pyrrolidin-1-ylsulfonyl)methyl)-1H-indol-3-yl)ethanamine; N,N-dimethyl-2-[5-(pyrrolidin-1-ylsulfonylmethyl)-1H-indol-3-yl]ethanamine; N,N-dimethyl-2-{5-[(pyrrolidin-1-ylsulfonyl)methyl]-1H-indol-3-yl}ethanamine; NCGC00095135-01; UNII-1O4XL5SN61; WKEMJKQOLOHJLZ-UHFFFAOYSA-N; 1-(((3-(2-(Dimethylamino)ethyl)indol-5-yl)methyl)sulfonyl)pyrrolidine; 154323-57-6; 1O4XL5SN61; 1-(((3-(2-(Dimethylamino)ethyl)-1H-indol-5-yl)methyl)sulfonyl)pyrrolidine, hydroxybutanedionate (1:1); 1-(((3-(2-(Dimethylamino)ethyl)indol-5-yl)methyl)sulfonyl)pyrrolidine malate (1:1); 1-[[[2-(Dimethyl-amino)ethyl]-1H-indol-5-yl]methyl]sulfonyl]pyrrolidine Malate; 181183-52-8; AK110510
|
| Cross-matching ID |
- PubChem CID
- 123607
- ChEBI ID
-
- CAS Number
-
- Formula
- C21H31N3O7S
- Canonical SMILES
- CN(C)CCC1=CNC2=C1C=C(C=C2)CS(=O)(=O)N3CCCC3.C(C(C(=O)O)O)C(=O)O
- InChI
- 1S/C17H25N3O2S.C4H6O5/c1-19(2)10-7-15-12-18-17-6-5-14(11-16(15)17)13-23(21,22)20-8-3-4-9-20;5-2(4(8)9)1-3(6)7/h5-6,11-12,18H,3-4,7-10,13H2,1-2H3;2,5H,1H2,(H,6,7)(H,8,9)
- InChIKey
- QHATUKWEVNMHRY-UHFFFAOYSA-N
|