| Cross-matching ID |
- PubChem CID
- 5281004
- PubChem SID
-
855541
; 7847312
; 7978823
; 8616471
; 10321783
; 11466546
; 11467666
; 11486132
; 11528595
; 12013315
; 14807548
; 14929955
; 17404737
; 24278277
; 39289945
; 46504869
; 47646553
; 47720602
; 47869643
; 47943751
; 48169361
; 49698535
; 50105232
; 53777280
; 53789711
; 56394980
; 56422168
; 57357962
; 77787022
; 81092787
; 85787937
; 90341081
; 90473637
; 92125441
; 92303636
; 92721807
; 93576669
; 103455384
; 103914268
; 104170124
; 121360873
; 121362487
; 121363112
; 124749512
; 124800069
; 124886759
; 126627527
; 126651544
; 127278596
; 127278597
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y7IU
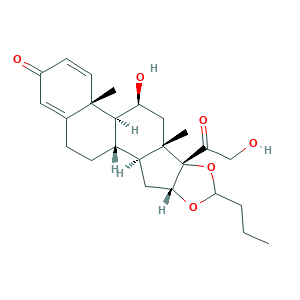
- Formula
- C25H34O6
- Canonical SMILES
- CCCC1OC2CC3C4CCC5=CC(=O)C=CC5(C4C(CC3(C2(O1)C(=O)CO)C)O)C
- InChI
- 1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21?,22+,23-,24-,25+/m0/s1
- InChIKey
- VOVIALXJUBGFJZ-KWVAZRHASA-N
|