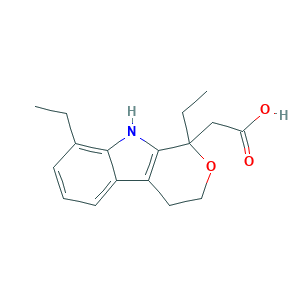
| Synonyms |
Edolan; Etodolaco; Etodolaco [INN-Spanish]; Etodolacum; Etodolacum [INN-Latin]; Etodolic acid; Lodine; Lodine XL; N-Methyl Etodolac; Ramodar; Ultradol; etodolac; 1,3,4,9-Tetrahydro-1,8-diethylpyrano(3,4-b)indole-1-acetic acid; 1,8-Diethyl-1,3,4,9-tetrahydropyrano(3,4-b)indole-1-acetic acid; 1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indole-1-acetic acid; 2-(1,8-diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)acetic acid; 41340-25-4; AY 24236; AY-24,236; AY-24236; CCRIS 3923; CHEMBL622; NSC 282126
|
| Cross-matching ID |
- PubChem CID
- 3308
- PubChem SID
-
9204
; 143623
; 224459
; 855612
; 5268437
; 7847381
; 7979205
; 8149693
; 8152106
; 10321309
; 11335322
; 11360561
; 11364138
; 11366700
; 11369262
; 11372457
; 11373819
; 11377424
; 11461533
; 11466259
; 11467379
; 11485110
; 11485958
; 11489203
; 11491244
; 11492225
; 11495058
; 12012673
; 14824710
; 17405042
; 24278407
; 26612147
; 26680024
; 26747213
; 26747214
; 29222445
; 46505184
; 47885271
; 47959585
; 47959586
; 48034967
; 48110316
; 48184861
; 48259079
; 48334348
; 48413770
; 49698757
; 49857374
; 50105240
; 50105241
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0N1WU
- Formula
- C17H21NO3
- Canonical SMILES
- CCC1=C2C(=CC=C1)C3=C(N2)C(OCC3)(CC)CC(=O)O
- InChI
- 1S/C17H21NO3/c1-3-11-6-5-7-12-13-8-9-21-17(4-2,10-14(19)20)16(13)18-15(11)12/h5-7,18H,3-4,8-10H2,1-2H3,(H,19,20)
- InChIKey
- NNYBQONXHNTVIJ-UHFFFAOYSA-N
|