| Cross-matching ID |
- PubChem CID
- 36462
- PubChem SID
-
4733
; 597749
; 7847193
; 7979208
; 8145856
; 8174504
; 11110521
; 12013288
; 14886949
; 14935915
; 24278178
; 24769897
; 34677904
; 46386954
; 46500626
; 46505434
; 47362874
; 48182835
; 48332078
; 49681767
; 49699016
; 49855364
; 53787668
; 56463308
; 57311878
; 71825014
; 75974183
; 85148351
; 85788790
; 89850276
; 92308660
; 92308790
; 99437037
; 104019076
; 104234176
; 104324084
; 117664408
; 117682510
; 119525100
; 121363248
; 124349593
; 124659141
; 124757097
; 124800231
; 124893613
; 125163901
; 127298729
; 127298730
; 127298731
; 127298732
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0B7EB
- Formula
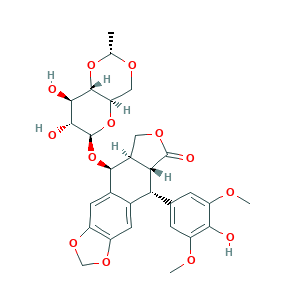
- C29H32O13
- Canonical SMILES
- CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O
- InChI
- 1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
- InChIKey
- VJJPUSNTGOMMGY-MRVIYFEKSA-N
|