Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0803) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Halothane
|
|||||
| Synonyms |
Halotan; Halotano; Halotano [INN-Spanish]; Halothan; Halothanum; Halothanum [INN-Latin]; Halsan; Narcotan; Narcotane; Narkotan; Alotano; Alotano [DCIT]; Anestan; Bromchlortrifluoraethanum; Bromochlorotrifluoroethane; Chalothane; Fluktan; Fluorotane; Fluothane; Freon 123B1; Ftorotan; Ftorotan [Russian]; Ftuorotan; Phthorothanum; Rhodialothan; halothane; 1,1,1-Trifluoro-2-chloro-2-bromoethane; 1-Bromo-1-chloro-2,2,2-trifluoroethane; 151-67-7; 2,2,2-Trifluoro-1-chloro-1-bromoethane; 2-BROMO-2-CHLORO-1,1,1-TRIFLUOROETHANE; Halan
|
|||||
| Indication | Anaesthesia [ICD11: 8E22] | Approved | [1] | |||
| Structure |
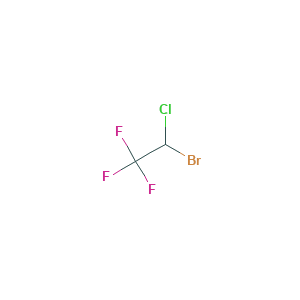 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 197.38 | Topological Polar Surface Area | 0 | ||
| Heavy Atom Count | 7 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

