Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0917) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Lapatinib ditosylate
|
|||||
| Synonyms |
Lapatinib (GW-572016) Ditosylate; Lapatinib (ditosylate); Lapatinib Ditosylate; Tykerb Ditosylate; 388082-77-7; 4WK72K94MC; AK-47669; GW-57201; GW-572016F Ditosylate; N-(3-CHLORO-4-((3-FLUOROBENZYL)OXY)PHENYL)-6-(5-(((2-(METHYLSULFONYL)ETHYL)AMINO)METHYL)FURAN-2-YL)QUINAZOLIN-4-AMINE BIS(4-METHYLBENZENESULFONATE); N-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-(5-{[(2-methanesulfonylethyl)amino]methyl}furan-2-yl)quinazolin-4-amine; UNII-4WK72K94MC; bis(4-methylbenzene-1-sulfonic acid)
|
|||||
| Indication | Breast cancer [ICD11: 2C60] | Approved | [1] | |||
| Structure |
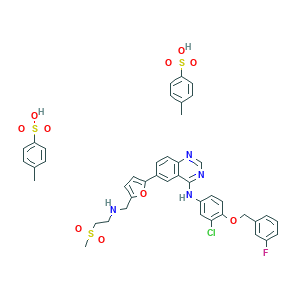 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 925.5 | Topological Polar Surface Area | 240 | ||
| Heavy Atom Count | 62 | Rotatable Bond Count | 13 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 15 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Lapatinib Ditosylate was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Detoxication versus Bioactivation Pathways of Lapatinib In Vitro: UGT1A1 Catalyzes the Hepatic Glucuronidation of Debenzylated Lapatinib Drug Metab Dispos. 2021 Mar;49(3):233-244. doi: 10.1124/dmd.120.000236. | |||||
| 3 | Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009 Dec;35(8):692-706. | |||||
| 4 | Mechanism-based inactivation of cytochrome P450 3A4 by lapatinib. Mol Pharmacol. 2010 Oct;78(4):693-703. | |||||
| 5 | Metabolic intermediate complex formation of human cytochrome P450 3A4 by lapatinib Drug Metab Dispos. 2011 Jun;39(6):1022-30. doi: 10.1124/dmd.110.037531. | |||||
| 6 | Case Study 11: Considerations for Enzyme Mapping Experiments-Interaction Between the Aldehyde Oxidase Inhibitor Hydralazine and Glutathione. Methods Mol Biol. 2021;2342:809-823. doi: 10.1007/978-1-0716-1554-6_30. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

