| General Information of Drug (ID:
DR1127) |
| Drug Name |
Naloxone
|
| Synonyms |
Nalone; Nalossone; Nalossone [DCIT]; Naloxona; Naloxona [INN-Spanish]; Naloxone [INN:BAN]; Naloxonum; Naloxonum [INN-Latin]; Narcan; Narcanti; Narcon; l-Naloxone; n-Allylnoroxymorphone; naloxone; (-)-Naloxone; 1-N-Allyl-14-hydroxynordihydromorphinone; EN 1530 base; N-Allyl-noroxymorphone; 1-N-Allyl-7,8-dihydro-14-hydroxynormorphinone; 17-Allyl-4,5-alpha-epoxy-3,14-dihydroxymorphinan-6-one; 17-Allyl-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one; 465-65-6; HSDB 3279; UNII-36B82AMQ7N; l-N-Allyl-14-hydroxynordihydromorphinone
|
| Indication |
Opiate dependence
[ICD11: 6C43]
|
Approved
|
[1]
|
| Structure |
|
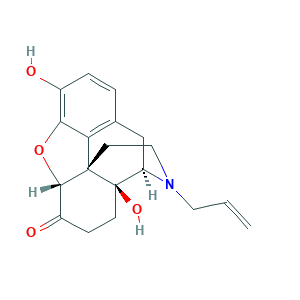 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
327.4 |
Topological Polar Surface Area |
70 |
| Heavy Atom Count |
24 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 5284596
- PubChem SID
-
9461
; 113816
; 841983
; 7980073
; 11039368
; 11466139
; 11467259
; 11485797
; 14777335
; 14924176
; 16463984
; 25663897
; 39317902
; 46508816
; 47588898
; 47588899
; 47662178
; 47736369
; 47959634
; 48334383
; 48416308
; 49698350
; 49993151
; 50070720
; 50104484
; 53788675
; 56312594
; 56312596
; 56352924
; 57288792
; 57359133
; 74711224
; 85787517
; 92309116
; 93166268
; 96024937
; 103170037
; 103915330
; 124886932
; 126688739
; 129442197
; 134337424
; 134974167
; 135650684
; 135651185
; 137001357
; 139076562
; 144205559
; 160964517
; 162183031
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0RN5A
- Formula
- C19H21NO4
- Canonical SMILES
- C=CCN1CCC23C4C(=O)CCC2(C1CC5=C3C(=C(C=C5)O)O4)O
- InChI
- 1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1
- InChIKey
- UZHSEJADLWPNLE-GRGSLBFTSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


