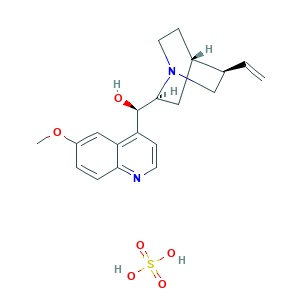
| Synonyms |
Quinine bisulfate; Quinine bisulfate [NF]; Quinine hydrogen sulfate; Quinine hydrogen sulphate; Quinine, sulfate (1:1) (salt); Qualaquin; Quinine; Quinine HCl; Quinine anhydrous; Quinine sulphate; Quinine, Anhydrous; Quinine, tannate; Quinineanhydrous; Quinoline alkaloid; UNII-A7V27PHC7A; chininum; quinina; quinine bisulphate; (-)-Quinine; (8S,9R)-Quinine; (R)-(-)-quinine; 130-95-0; 6'-Methoxycinchonan-9-ol; 6'-Methoxycinchonidine; 6'-Methoxycinchonine; 6-Methoxycinchonine; A7V27PHC7A; Aflukin; CHEBI:15854; Chinin; Chinin [German]; Chinine; Cinchonan-9-ol, 6'-methoxy-, (8a,9R)-; Coco-Quinine; LOUPRKONTZGTKE-WZBLMQSHSA-N; MFCD00198096; NSC 192949; SCHEMBL29712; UNII-M201HC068W; (-)-Quinine Sulfate Dihydrate; (R)-(6-Methoxyquinolin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methanol sulfate; 549-56-4; 6119-70-6; 804-63-7; 8alpha,9R-6'-Methoxycinchonan-9-ol, sulfate (1:1) salt; CHEMBL1201100; EC 208-970-9; EINECS 208-970-9; FEMA No. 2975; GNF-Pf-506; M201HC068W; QUININE SULFATE DIHYDRATE
|
| Cross-matching ID |
- PubChem CID
- 11949689
- CAS Number
-
- TTD Drug ID
- D03DDR
- Formula
- C20H26N2O6S
- Canonical SMILES
- COC1=CC2=C(C=CN=C2C=C1)C(C3CC4CCN3CC4C=C)O.OS(=O)(=O)O
- InChI
- 1S/C20H24N2O2.H2O4S/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18;1-5(2,3)4/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3;(H2,1,2,3,4)/t13-,14-,19-,20+;/m0./s1
- InChIKey
- AKYHKWQPZHDOBW-DSXUQNDKSA-N
|