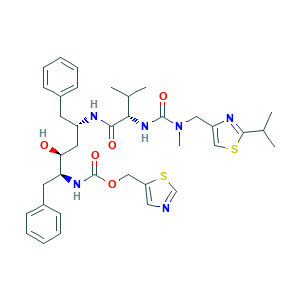
| Synonyms |
Ritonavir, 98%; ABBOTT-84538; ABT 538; ABT-538; Abbott 84538; CHEBI:45409; DRG-0244; Norvir; Norvir Softgel; O3J8G9O825; ritonavir; 155213-67-5; 5-Thiazolylmethyl ((alphaS)-alpha-((1S,3S-1-hydroxy-3-((2S)-2-(3-((2-isopropyl-4-thiazolyl)methyl)-3-methylureido)-3-methylbutyramido)-4-phenylbutyl)phenethyl)carbamate; A-84538; DSSTox_CID_28553; DSSTox_GSID_48627; DSSTox_RID_82825; HSDB 7160; MFCD00927142; NCGC00159462-02; NCGC00183130-01; NSC693184; RTV; UNII-O3J8G9O825
|
| Cross-matching ID |
- PubChem CID
- 392622
- PubChem SID
-
9449
; 523930
; 583815
; 612199
; 822215
; 827183
; 855141
; 7847493
; 7890279
; 7980525
; 8030461
; 10279496
; 11528746
; 12014859
; 14766505
; 14790837
; 17422094
; 26719904
; 29215414
; 46386817
; 46392172
; 46393138
; 46505050
; 49681649
; 53789763
; 57402349
; 71825024
; 79712259
; 81092847
; 92308258
; 92711419
; 93166545
; 93167041
; 99436927
; 103244854
; 104178993
; 104616751
; 104829353
; 117877983
; 118048821
; 124658994
; 124757062
; 124801360
; 124876828
; 124892213
; 124894323
; 125163866
; 125333191
; 126592976
; 126630875
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0ZU9R
- Formula
- C37H48N6O5S2
- Canonical SMILES
- CC(C)C1=NC(=CS1)CN(C)C(=O)NC(C(C)C)C(=O)NC(CC2=CC=CC=C2)CC(C(CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)O
- InChI
- 1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
- InChIKey
- NCDNCNXCDXHOMX-XGKFQTDJSA-N
|