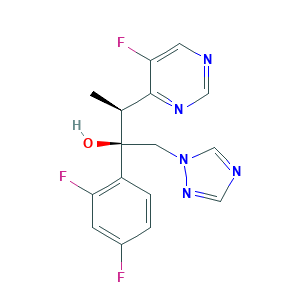
| Synonyms |
Voriconazol; Voriconazole; Voriconazole [USAN:INN:BAN]; Voriconazolum; JFU09I87TR; UK 109496; UK-109,496; UK-109496; (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol; (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol; (alphaR,betaS)-alpha-(2,4-Difluorophenyl)-5-fluoro-beta-methyl-alpha-(1H-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol; 137234-62-9; C16H14F3N5O; CHEBI:10023; CHEMBL638; CPD000466350; DRG-0301; UNII-JFU09I87TR; VCZ; VRC; Vfend
|
| Cross-matching ID |
- PubChem CID
- 71616
- PubChem SID
-
9824
; 618929
; 7847644
; 8194731
; 12014673
; 14754154
; 14900759
; 26719857
; 43127923
; 46386721
; 46506421
; 49681678
; 49830865
; 50065685
; 53790739
; 57318277
; 58107314
; 79258320
; 92308499
; 92712543
; 93165418
; 99437153
; 103042056
; 103188976
; 104350892
; 109692905
; 117510444
; 118048601
; 121361066
; 124658937
; 124757251
; 124766492
; 125083219
; 125164055
; 126592905
; 126625471
; 126656629
; 126670609
; 127494461
; 134338507
; 135029377
; 136340251
; 136367733
; 137006421
; 140298163
; 144076218
; 144205794
; 152164501
; 160870955
; 160963927
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0N3VR
- Formula
- C16H14F3N5O
- Canonical SMILES
- CC(C1=NC=NC=C1F)C(CN2C=NC=N2)(C3=C(C=C(C=C3)F)F)O
- InChI
- 1S/C16H14F3N5O/c1-10(15-14(19)5-20-7-22-15)16(25,6-24-9-21-8-23-24)12-3-2-11(17)4-13(12)18/h2-5,7-10,25H,6H2,1H3/t10-,16+/m0/s1
- InChIKey
- BCEHBSKCWLPMDN-MGPLVRAMSA-N
|