| DME Name |
DME Info |
Species |
Uniprot ID |
EC Number |
REF |
|
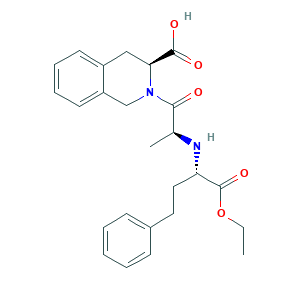
Carboxylesterase 1 (CES1)
|
DME0054
|
Homo sapiens
|
|
|
[2]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1251
|
Bacteroides fragilis
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1254
|
Bacteroides thetaiotaomicron
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1256
|
Bacteroides sartorii
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1363
|
Bacteroides cellulosilyticus
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1364
|
Bacteroides coprophilus
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1365
|
Bacteroides dorei
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1366
|
Bacteroides eggerthii
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1375
|
Bacteroides intestinalis
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1377
|
Bacteroides pectinophilus
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1379
|
Bacteroides stercoris
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1384
|
Bacteroides vulgatus
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1385
|
Bacteroides xylanisolvens
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1388
|
Bifidobacterium breve
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1391
|
Bifidobacterium ruminatum
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1393
|
Blautia hansenii
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1402
|
Clostridium botulinum
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1431
|
Holdemanella biformis
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1439
|
Marvinbryantia formatexigens
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1444
|
Odoribacter splanchnicus
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1445
|
Parabacteroides distasonis
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1449
|
Prevotella copri
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1462
|
Salmonella enterica
|
Not Available |
Not Available |
[3]
|
|
Unclear metabolic mechanism (DME-unclear)
|
DME1467
|
Subdoligranulum variabile
|
Not Available |
Not Available |
[3]
|
|
|
|
|
|
|