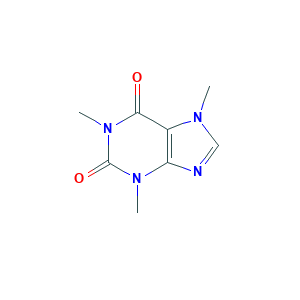
| Synonyms |
Cafeina; Caffedrine; Caffein; Caffeine, synthetic; Caffine; Cafipel; Coffein; Coffeine; Coffeinum; Caffeine citrate; DHCplus; Dexitac; Durvitan; Eldiatric C; Guaranine; Hycomine; Koffein; Mateina; Methyltheobromide; Methyltheobromine; Nix Nap; No-Doz; Nodaca; Organex; Phensal; Quick-Pep; Refresh'n; Stim; Thein; Theine; Theophylline, 7-methyl; Tirend; Vivarin; Wigraine; caffeine; Caffeina citrate; Caffeine citrate (1:1); Caffeine citrated; Caffeine, Citrated; Citrated caffein; Citric acid, compd. with caffeine (1:1); DSSTox_CID_26938; DSSTox_GSID_46938; DSSTox_RID_82023; NCGC00015208-09; U26EO4675Q; UNII-U26EO4675Q; 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, mixt. with 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione; 1,2,3-Propanetricarboxylic acid,2-hydroxy-,compounds,mixt. with 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6- dione; 69-22-7; C8H10N4O2.C6H8O7; CAS-69-22-7; 1,3,7-Trimethyl-2,6-dioxopurine; 1,3,7-Trimethylxanthine; 1958/8/2; 3,7-Dihydro-1,3,7-trimethyl-1H-purine-2,6-dione; 7-Methyltheophylline; Alert-pep; Anhydrous caffeine; Cafamil; Cafecon
|