| General Information of Drug (ID:
DR0329) |
| Drug Name |
Cinnarizine
|
| Synonyms |
Cinarizina; Cinarizina [INN-Spanish]; Cinarizine; Cinazyn; Cinnageron; Cinnarizine [USAN:INN:BAN:JAN]; Cinnarizinum; Cinnarizinum [INN-Latin]; Aplactan; Dimitron; Dimitronal; Folcodal; Glanil; Labyrin; Lazeta; Marisan; Midronal; Mitronal; R 1575; Stugeron; Stutgeron; Stutgin; Toliman; cinnarizine; 1-(Diphenylmethyl)-4-(3-phenyl-2-propenyl)piperazine; 1-Benzhydryl-4-cinnamylpiperazin; 1-Cinnamyl-4-(diphenylmethyl)piperazine; 298-57-7; 516 MD; Piperazine, 1-(diphenylmethyl)-4-(3-phenyl-2-propenyl)-; R 516; Sepan; UNII-3DI2E1X18L
|
| Indication |
Haemorrhagic stroke
[ICD11: 8B20]
|
Phase 4
|
[1]
|
| Structure |
|
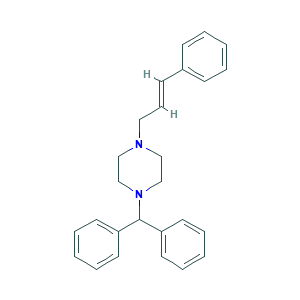 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
368.5 |
Topological Polar Surface Area |
6.5 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
0 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 1547484
- PubChem SID
-
145189
; 597357
; 855570
; 7848358
; 7978948
; 8652487
; 10321216
; 10517604
; 10852043
; 14803894
; 17404877
; 24278320
; 32247729
; 47216936
; 47440424
; 47515486
; 47810927
; 47810928
; 48110619
; 48415781
; 49698416
; 50012381
; 50103929
; 50103930
; 50103931
; 50140413
; 53777420
; 56422441
; 56464175
; 57288760
; 57409047
; 81093277
; 85148348
; 85173395
; 85209898
; 85231348
; 85788038
; 89099692
; 89850280
; 90340734
; 92125122
; 92303637
; 103224720
; 103913696
; 103928925
; 106681504
; 110522301
; 117706973
; 121361114
; 121362793
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Q3YO
- Formula
- C26H28N2
- Canonical SMILES
- C1CN(CCN1CC=CC2=CC=CC=C2)C(C3=CC=CC=C3)C4=CC=CC=C4
- InChI
- 1S/C26H28N2/c1-4-11-23(12-5-1)13-10-18-27-19-21-28(22-20-27)26(24-14-6-2-7-15-24)25-16-8-3-9-17-25/h1-17,26H,18-22H2/b13-10+
- InChIKey
- DERZBLKQOCDDDZ-JLHYYAGUSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


