| General Information of Drug (ID:
DR1520) |
| Drug Name |
Suprofen
|
| Synonyms |
Sulproltin; Suprocil; Suprofen (Profenal); Suprofene; Suprofene [INN-French]; Suprofenum; Suprofenum [INN-Latin]; Suprol; Sutoprofen; TN 762; Maldocil; Masterfen; Profenal; R 25061; R-25,061; R-25061; Srendam; Topalgic; p-2-Thenoylhydratropic acid; suprofen; (+-)-2-(p-(2-Thenoyl)phenyl)propionic acid; 2-(4-(2-Thenoyl)phenyl)propionsaeure; 2-[4-(thiophene-2-carbonyl)phenyl]propanoic acid; 4-(2-Thenoyl)hydratropsaeure; 40828-46-4; C14H12O3S; CHEBI:9362; NSC 303611; alpha-Methyl-4-(2-thienylcarbonyl)benzeneacetic acid
|
| Indication |
Iris sphincter disorder
[ICD11: 9B01]
|
Approved
|
[1]
|
| Structure |
|
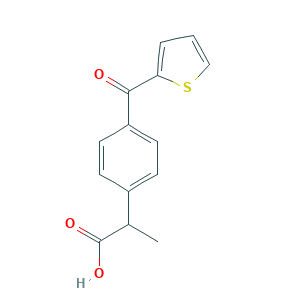 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
260.31 |
Topological Polar Surface Area |
82.6 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 5359
- PubChem SID
-
9528
; 224830
; 453324
; 3180764
; 7672824
; 7847518
; 8149755
; 8153294
; 10321232
; 11335651
; 11360890
; 11364051
; 11366613
; 11369175
; 11372035
; 11374691
; 11377337
; 11461862
; 11466844
; 11467964
; 11485276
; 11486368
; 11489246
; 11490743
; 11492841
; 11494971
; 12012657
; 14848020
; 26612210
; 26679843
; 26748525
; 26748526
; 29224411
; 46508210
; 47365135
; 47662231
; 47736424
; 47810708
; 47810709
; 48035064
; 49698781
; 49977399
; 50025833
; 50107425
; 56459397
; 56463227
; 57322736
; 74790379
; 85787329
; 92124483
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D07BPS
- Formula
- C14H12O3S
- Canonical SMILES
- CC(C1=CC=C(C=C1)C(=O)C2=CC=CS2)C(=O)O
- InChI
- 1S/C14H12O3S/c1-9(14(16)17)10-4-6-11(7-5-10)13(15)12-3-2-8-18-12/h2-9H,1H3,(H,16,17)
- InChIKey
- MDKGKXOCJGEUJW-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


