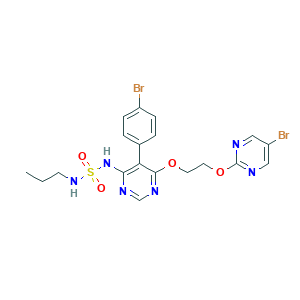
| Cross-matching ID |
- PubChem CID
- 16004692
- PubChem SID
-
24380756
; 28680076
; 37128307
; 135262332
; 135626853
; 136023841
; 139257646
; 152256091
; 152258558
; 160647393
; 160703277
; 162202062
; 163344650
; 171572070
; 172087019
; 172096639
; 174007274
; 175427162
; 178103924
; 184815478
; 189628674
; 198971703
; 198993773
; 223385903
; 223701845
; 223705199
; 227656403
; 241382239
; 242590074
; 248261348
; 249867527
; 250225136
; 252069311
; 252110242
; 252166931
; 252225701
; 252437394
; 252451827
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0S7JH
- Formula
- C19H20Br2N6O4S
- Canonical SMILES
- CCCNS(=O)(=O)NC1=C(C(=NC=N1)OCCOC2=NC=C(C=N2)Br)C3=CC=C(C=C3)Br
- InChI
- 1S/C19H20Br2N6O4S/c1-2-7-26-32(28,29)27-17-16(13-3-5-14(20)6-4-13)18(25-12-24-17)30-8-9-31-19-22-10-15(21)11-23-19/h3-6,10-12,26H,2,7-9H2,1H3,(H,24,25,27)
- InChIKey
- JGCMEBMXRHSZKX-UHFFFAOYSA-N
|