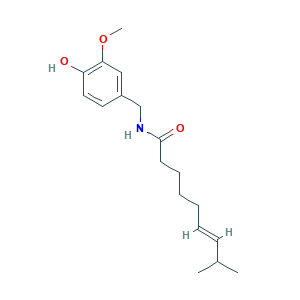
| Synonyms |
Capsaicin; Capsaicin (JAN/USP); Capsaicin [USAN]; Axsain; CAPSAICINE; E-CAPSAICIN; Isodecenoic acid vanillylamide; NGX-4010; Qutenza; Styptysat; Transacin; Vanilloid; ZOSTRIX (TN); Zostrix; trans-Capsaicin; (E)-8-Methyl-N-vanillyl-6-nonenamide; (E)-Capsaicin; 404-86-4; 6-Nonenamide, 8-methyl-N-vanillyl-, (E)-; 8-Methyl-N-Vanillyl-6-Nonenamide; 8-Methyl-N-vanillyl-trans-6-nonenamide; C18H27NO3; CCRIS 1588; CHEBI:3374; Caswell No. 158; FEMA No. 3404; HSDB 954; NCI-C56564; NSC 56353; UNII-S07O44R1ZM; trans-8-Methyl-N-vanillyl-6-nonenamide
|
| Cross-matching ID |
- PubChem CID
- 1548943
- PubChem SID
-
9083
; 105773
; 853800
; 1916595
; 7847316
; 7978850
; 8149724
; 8653307
; 10321304
; 11537699
; 12015395
; 14923155
; 17389996
; 17486482
; 22391501
; 24847432
; 24853014
; 24853015
; 24862158
; 24896598
; 26612181
; 26680132
; 26681474
; 26744231
; 26747263
; 26747264
; 26752818
; 26752819
; 26752820
; 26758379
; 29203915
; 32248886
; 46487934
; 47216576
; 47364983
; 47515116
; 47662075
; 47662076
; 47736251
; 47736252
; 47810544
; 47885207
; 48034884
; 48259019
; 48413373
; 48423080
; 48424925
; 49688535
; 49698675
; 49747048
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U5CE
- Formula
- C18H27NO3
- Canonical SMILES
- CC(C)C=CCCCCC(=O)NCC1=CC(=C(C=C1)O)OC
- InChI
- 1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+
- InChIKey
- YKPUWZUDDOIDPM-SOFGYWHQSA-N
|