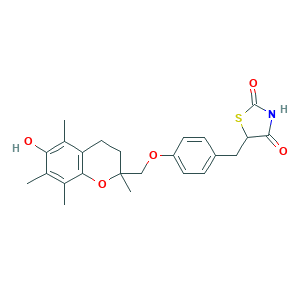
| Synonyms |
Troglitazone (CS-045); troglitazone; (+-)-all-rac-5-(p-((6-Hydroxy-2,5,7,8-tetramethyl-2-chromanyl)methoxy)benzyl)-2,4-thiazolidinedione; Noscal; Prelay; Rezulin; Rezulin (TN); Romglizone; Romozin; 5-(4-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl)thiazolidine-2,4-dione; 97322-87-7; BRN 4338399; C24H27NO5S; CCRIS 8969; CHEBI:9753; CI 991; CI-991; CS 045; CS-045; CS045; GR 92132X; GR-92132X; GR92132X; GXPHKUHSUJUWKP-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 5591
- PubChem SID
-
841060
; 4894294
; 7847461
; 7980843
; 8153443
; 11533348
; 12013560
; 14905963
; 22391425
; 24724629
; 26681461
; 26755242
; 26755243
; 26757864
; 26759664
; 29216382
; 29224629
; 46487963
; 46504655
; 47365443
; 47811013
; 48035383
; 48416678
; 50052241
; 53788686
; 56311256
; 56312004
; 56313972
; 56313996
; 57288866
; 57322861
; 74940595
; 85246179
; 85257254
; 85787118
; 91011836
; 92307894
; 92308758
; 103164301
; 103853949
; 104309674
; 104829539
; 119526104
; 124892345
; 124892346
; 124892347
; 124892348
; 125334732
; 126647230
; 126683373
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06XZW
- Formula
- C24H27NO5S
- Canonical SMILES
- CC1=C(C2=C(CCC(O2)(C)COC3=CC=C(C=C3)CC4C(=O)NC(=O)S4)C(=C1O)C)C
- InChI
- 1S/C24H27NO5S/c1-13-14(2)21-18(15(3)20(13)26)9-10-24(4,30-21)12-29-17-7-5-16(6-8-17)11-19-22(27)25-23(28)31-19/h5-8,19,26H,9-12H2,1-4H3,(H,25,27,28)
- InChIKey
- GXPHKUHSUJUWKP-UHFFFAOYSA-N
|