| Cross-matching ID |
- PubChem CID
- 441207
- PubChem SID
-
9170
; 3139545
; 7847363
; 7979082
; 10298760
; 11533060
; 12015761
; 14889043
; 16335905
; 24434759
; 24893976
; 26754429
; 29204037
; 36885098
; 46506035
; 47349645
; 48169692
; 48318721
; 48394206
; 48415893
; 48496158
; 50109842
; 50333813
; 53787897
; 57287891
; 57403584
; 85300744
; 85842716
; 87567202
; 92298457
; 103556970
; 104624909
; 124573763
; 127284350
; 127284351
; 127303961
; 127303962
; 127303963
; 127303964
; 127303965
; 127303966
; 127303967
; 127303968
; 127303969
; 127303970
; 134337444
; 134972304
; 135377837
; 135756584
; 137004198
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M3QP
- Formula
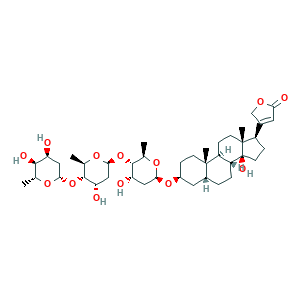
- C41H64O13
- Canonical SMILES
- CC1C(C(CC(O1)OC2C(OC(CC2O)OC3C(OC(CC3O)OC4CCC5(C(C4)CCC6C5CCC7(C6(CCC7C8=CC(=O)OC8)O)C)C)C)C)O)O
- InChI
- 1S/C41H64O13/c1-20-36(46)29(42)16-34(49-20)53-38-22(3)51-35(18-31(38)44)54-37-21(2)50-33(17-30(37)43)52-25-8-11-39(4)24(15-25)6-7-28-27(39)9-12-40(5)26(10-13-41(28,40)47)23-14-32(45)48-19-23/h14,20-22,24-31,33-38,42-44,46-47H,6-13,15-19H2,1-5H3/t20-,21-,22-,24-,25+,26-,27+,28-,29+,30+,31+,33+,34+,35+,36-,37-,38-,39+,40-,41+/m1/s1
- InChIKey
- WDJUZGPOPHTGOT-XUDUSOBPSA-N
|