| Synonyms |
Levometadona; Levometadona [INN-Spanish]; Levomethadone; Levomethadone (INN); Levomethadone [INN]; Levomethadonum; Levomethadonum [INN-Latin]; Levothyl; Polamivet; L-Polamidon; l-Polamivet; (-)-(R)-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; (-)-Methadone; (6R)-Methadone; (R)-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; (R)-Methadone; 125-58-6; 3-HEPTANONE, 6-(DIMETHYLAMINO)-4,4-DIPHENYL-, L-; 3-Hetpanone, 6-(dimethylamino)-4,4-diphenyl-, (R)- (9CI); 6Y75Z4E8NS; L-6-(Dimethylamino)-4,4-diphenyl-3-heptanone; UNII-6Y75Z4E8NS
|
| Cross-matching ID |
- PubChem CID
- 22267
- PubChem SID
-
841133
; 8166385
; 16828013
; 29289580
; 47359869
; 47510027
; 50251534
; 57331123
; 93167208
; 96024811
; 103412836
; 104355526
; 129415111
; 131326064
; 135650582
; 138237674
; 141601059
; 144239892
; 162222373
; 175271129
; 198945476
; 226513694
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09OJQ
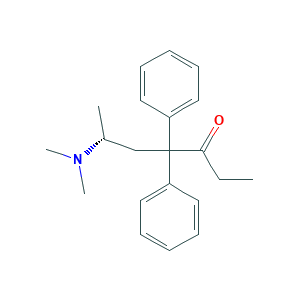
- Formula
- C21H27NO
- Canonical SMILES
- CCC(=O)C(CC(C)N(C)C)(C1=CC=CC=C1)C2=CC=CC=C2
- InChI
- 1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3/t17-/m1/s1
- InChIKey
- USSIQXCVUWKGNF-QGZVFWFLSA-N
|