| General Information of Drug (ID:
DR1765) |
| Drug Name |
Bromfenac
|
| Synonyms |
BromSite; Bromday; Bromfenac; Bromfenac (INN); Bromfenac [INN]; Bromfenaco; Bromfenaco [Spanish]; Bromfenacum; Bromfenacum [Latin]; Duract; ISV-303; Prolensa; Xibrom; 120638-55-3; 2-(2-Amino-3-(4-bromobenzoyl)phenyl)acetic acid; 2-Amino-3-(4-bromobenzoyl)benzeneacetic acid; 2-[2-amino-3-(4-bromobenzoyl)phenyl]acetic acid; 4mjq; 864P0921DW; 91714-94-2; AHR 10282; AHR-10282; AHR-10282B; AK-85648; Benzeneacetic acid, 2-amino-3-(4-bromobenzoyl)-; C15H12BrNO3; CHEBI:240107; UNII-864P0921DW; [2-amino-3-(4-bromobenzoyl)phenyl]acetic acid
|
| Indication |
Cataract
[ICD11: 9B10]
|
Approved
|
[1]
|
| Structure |
|
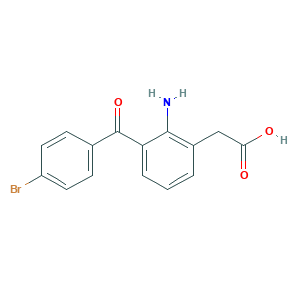 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
334.16 |
Topological Polar Surface Area |
80.4 |
| Heavy Atom Count |
20 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 60726
- PubChem SID
-
8187024
; 14802056
; 43118081
; 46508121
; 50065379
; 50714597
; 51091863
; 53786963
; 57288753
; 57314080
; 85433311
; 103293849
; 104321485
; 117562408
; 119526091
; 125536482
; 129879365
; 131330262
; 134338019
; 135065652
; 137005805
; 142970983
; 152227509
; 160814551
; 160964302
; 162197012
; 163089933
; 163373042
; 163837609
; 164117525
; 164175212
; 172092503
; 175268177
; 178103707
; 179151433
; 184545820
; 186004941
; 196396861
; 203081294
; 204420653
; 223388494
; 223435566
; 223484710
; 223551046
; 223656326
; 224730616
; 226420876
; 242065764
; 249847324
; 250181405
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U1OM
- Formula
- C15H12BrNO3
- Canonical SMILES
- C1=CC(=C(C(=C1)C(=O)C2=CC=C(C=C2)Br)N)CC(=O)O
- InChI
- 1S/C15H12BrNO3/c16-11-6-4-9(5-7-11)15(20)12-3-1-2-10(14(12)17)8-13(18)19/h1-7H,8,17H2,(H,18,19)
- InChIKey
- ZBPLOVFIXSTCRZ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


