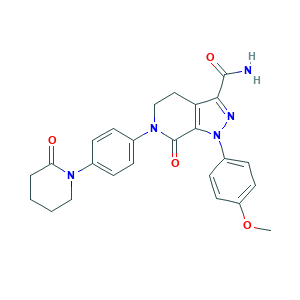
| Synonyms |
Apixaban; Apixaban(BMS-562247-01); Eliquis; apixabanum; 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide; 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl)phenyl)-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide; 3Z9Y7UWC1J; 503612-47-3; BMS 562247-01; BMS-562247; BMS-562247-01; BMS562247-01; CHEBI:72296; CHEMBL231779; UNII-3Z9Y7UWC1J
|
| Cross-matching ID |
- PubChem CID
- 10182969
- PubChem SID
-
15178464
; 17397366
; 22578033
; 26736936
; 40272539
; 46519072
; 51992439
; 53812906
; 56310620
; 57374335
; 99375349
; 99444299
; 103535612
; 104253239
; 109693404
; 124757362
; 124772059
; 125164166
; 125433570
; 126644898
; 126665798
; 134339057
; 135252374
; 135727449
; 135727826
; 136367462
; 136368094
; 136920385
; 137183704
; 139455355
; 144115617
; 152258806
; 152343992
; 152344098
; 160647657
; 160874191
; 160962913
; 160968889
; 162011738
; 163884621
; 164194092
; 164777207
; 165247616
; 170497908
; 172912849
; 174006823
; 174549337
; 175266926
; 175427077
; 178103006
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0I5HF
- Formula
- C25H25N5O4
- Canonical SMILES
- COC1=CC=C(C=C1)N2C3=C(CCN(C3=O)C4=CC=C(C=C4)N5CCCCC5=O)C(=N2)C(=O)N
- InChI
- 1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32)
- InChIKey
- QNZCBYKSOIHPEH-UHFFFAOYSA-N
|