| Cross-matching ID |
- PubChem CID
- 2724385
- PubChem SID
-
9171
; 3139699
; 7847364
; 7979083
; 8787891
; 10321270
; 11466465
; 11467585
; 11486129
; 11533002
; 14840467
; 16531631
; 17389540
; 24893992
; 24894045
; 25664046
; 26752810
; 29204039
; 30082596
; 46508524
; 47277036
; 47350829
; 47500941
; 47871144
; 48415894
; 48425070
; 48493824
; 49698492
; 49718191
; 50105460
; 50105461
; 56313674
; 56422204
; 57287890
; 57409429
; 57654114
; 79412392
; 85788562
; 87568294
; 90481132
; 92125420
; 92298240
; 92729949
; 93167166
; 99431517
; 103707689
; 103913751
; 111366106
; 117393920
; 121363091
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02OZE
- Formula
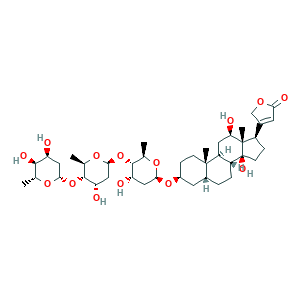
- C41H64O14
- Canonical SMILES
- CC1C(C(CC(O1)OC2C(OC(CC2O)OC3C(OC(CC3O)OC4CCC5(C(C4)CCC6C5CC(C7(C6(CCC7C8=CC(=O)OC8)O)C)O)C)C)C)O)O
- InChI
- 1S/C41H64O14/c1-19-36(47)28(42)15-34(50-19)54-38-21(3)52-35(17-30(38)44)55-37-20(2)51-33(16-29(37)43)53-24-8-10-39(4)23(13-24)6-7-26-27(39)14-31(45)40(5)25(9-11-41(26,40)48)22-12-32(46)49-18-22/h12,19-21,23-31,33-38,42-45,47-48H,6-11,13-18H2,1-5H3/t19-,20-,21-,23-,24+,25-,26-,27+,28+,29+,30+,31-,33+,34+,35+,36-,37-,38-,39+,40+,41+/m1/s1
- InChIKey
- LTMHDMANZUZIPE-PUGKRICDSA-N
|