Detail Information of Xenobiotics
| General Information of Xenobiotics (ID: XEO00090) | ||||||
|---|---|---|---|---|---|---|
| Xenobiotics Name |
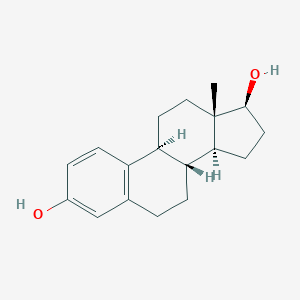
Estradiol
|
|||||
| Xenobiotics Type |
Pharmaceutical Agent(s)
|
|||||
| Classification |
Approved/Marketed Drug
|
|||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5757"></iframe>
|
 |
||||
| 3D MOL | 2D MOL | |||||
| PubChem CID | ||||||
| DME(s) Modulated by This Xenobiotics | ||||||
| DME(s) Inhibited by This Xenobiotics | ||||||
| Alcohol dehydrogenase class-I alpha (ADH1A) | DME Info | Homo sapiens | [1] | |||
| Alcohol dehydrogenase class-I beta (ADH1B) | DME Info | Homo sapiens | [1] | |||
| Alcohol dehydrogenase class-I gamma (ADH1C) | DME Info | Homo sapiens | [1] | |||
| Alcohol dehydrogenase class-II (ADH4) | DME Info | Homo sapiens | [1] | |||
| Alcohol dehydrogenase class-V (ADH6) | DME Info | Homo sapiens | [1] | |||
| Small intestine reductase (AKR1B10) | DME Info | Homo sapiens | [2] | |||
| Aldo-keto reductase 1C3 (AKR1C3) | DME Info | Homo sapiens | [3], [4] | |||
| Aldo-keto reductase 1C4 (AKR1C4) | DME Info | Homo sapiens | [1] | |||
| Aldo-keto reductase 1D1 (AKR1D1) | DME Info | Homo sapiens | [1], [5] | |||
| Porphobilinogen synthase (ALAD) | DME Info | Homo sapiens | [6], [7] | |||
| Aminomuconic semialdehyde dehydrogenase (ALDH12) | DME Info | Homo sapiens | [1] | |||
| Bile acid-CoA thioesterase (BAAT) | DME Info | Homo sapiens | [1], [5] | |||
| Catechol O-methyltransferase (COMT) | DME Info | Homo sapiens | [8], [9] | |||
| Cytochrome P450 1A1 (CYP1A1) | DME Info | Homo sapiens | [9] | |||
| Cytochrome P450 1A2 (CYP1A2) | DME Info | Homo sapiens | [10] | |||
| Cytochrome P450 1B1 (CYP1B1) | DME Info | Homo sapiens | [11] | |||
| Retinoic acid 4-hydroxylase 26B1 (CYP26B1) | DME Info | Homo sapiens | [3] | |||
| Cytochrome P450 2C8 (CYP2C8) | DME Info | Homo sapiens | [1] | |||
| Cytochrome P450 2F1 (CYP2F1) | DME Info | Homo sapiens | [12] | |||
| Cytochrome P450 3A7 (CYP3A7) | DME Info | Homo sapiens | [12], [1] | |||
| Cytochrome P450 4F2 (CYP4F2) | DME Info | Homo sapiens | [1] | |||
| Leukotriene B4 omega-hydroxylase (CYP4F3) | DME Info | Homo sapiens | [12] | |||
| Delta(24)-sterol reductase (DHCR24) | DME Info | Homo sapiens | [13], [14] | |||
| Cellular glutathione peroxidase (GPX1) | DME Info | Homo sapiens | [14] | |||
| Glutathione S-transferase alpha-1 (GSTA1) | DME Info | Homo sapiens | [4] | |||
| Glutathione S-transferase pi (GSTP1) | DME Info | Homo sapiens | [12] | |||
| Glutathione S-transferase theta-1 (GSTT1) | DME Info | Homo sapiens | [4] | |||
| Corticosteroid 11-beta-dehydrogenase 2 (HSD11B2) | DME Info | Homo sapiens | [15] | |||
| Estradiol 17-beta-dehydrogenase 2 (HSD17B2) | DME Info | Homo sapiens | [1], [5] | |||
| Keto-steroid reductase (HSD17B7) | DME Info | Homo sapiens | [4] | |||
| Iduronate 2-sulfatase (IDS) | DME Info | Homo sapiens | [16] | |||
| Monoamine oxidase type A (MAO-A) | DME Info | Homo sapiens | [17] | |||
| S-adenosylmethionine synthase 1 (MAT1A) | DME Info | Homo sapiens | [1] | |||
| Neprilysin (MME) | DME Info | Homo sapiens | [18] | |||
| Metallothionein-1A (MT1A) | DME Info | Homo sapiens | [12] | |||
| Metallothionein-2A (MT2A) | DME Info | Homo sapiens | [4], [12] | |||
| N-acetyltransferase 1 (NAT1) | DME Info | Homo sapiens | [4] | |||
| N-acetyltransferase 2 (NAT2) | DME Info | Homo sapiens | [1] | |||
| Quinone reductase 1 (NQO1) | DME Info | Homo sapiens | [19] | |||
| Ecto-5'-nucleotidase (NT5E) | DME Info | Homo sapiens | [20], [2] | |||
| Phosphodiesterase 5A (PDE5A) | DME Info | Homo sapiens | [21] | |||
| Serum paraoxonase/arylesterase 1 (PON1) | DME Info | Homo sapiens | [1] | |||
| Proline dehydrogenase 1 (PRODH) | DME Info | Homo sapiens | [4] | |||
| Putrescine acetyltransferase (SSAT1) | DME Info | Homo sapiens | [6] | |||
| Steroid 5-alpha-reductase 2 (SRD5A2) | DME Info | Homo sapiens | [1] | |||
| Sulfotransferase 1E1 (SULT1E1) | DME Info | Homo sapiens | [22] | |||
| Sulfotransferase 2A1 (SULT2A1) | DME Info | Homo sapiens | [1] | |||
| Tryptophan oxygenase (TRPO) | DME Info | Homo sapiens | [1] | |||
| Transglutaminase K (TGM1) | DME Info | Homo sapiens | [2] | |||
| Thiopurine methyltransferase (TPMT) | DME Info | Homo sapiens | [12] | |||
| UDP-glucuronosyltransferase 1A1 (UGT1A1) | DME Info | Homo sapiens | [23] | |||
| UDP-glucuronosyltransferase 1A3 (UGT1A3) | DME Info | Homo sapiens | [24] | |||
| UDP-glucuronosyltransferase 1A9 (UGT1A9) | DME Info | Homo sapiens | [23] | |||
| UDP-glucuronosyltransferase 2B4 (UGT2B4) | DME Info | Homo sapiens | [1] | |||
| DME(s) Induced by This Xenobiotics | ||||||
| Acetylcholinesterase (ACHE) | DME Info | Homo sapiens | [1] | |||
| Aldo-keto reductase 1C1 (AKR1C1) | DME Info | Homo sapiens | [3] | |||
| Aldo-keto reductase 1C2 (AKR1C2) | DME Info | Homo sapiens | [3] | |||
| Asparagine synthetase (ASNS) | DME Info | Homo sapiens | [1] | |||
| Cytosolic branched aminotransferase (BCAT1) | DME Info | Homo sapiens | [4] | |||
| Carbonic anhydrase II (CA2) | DME Info | Homo sapiens | [3], [4] | |||
| Choline dehydrogenase (CHDH) | DME Info | Homo sapiens | [4] | |||
| Cholesterol desmolase (CYP11A1) | DME Info | Homo sapiens | [25] | |||
| Aromatase (CYP19A1) | DME Info | Homo sapiens | [26] | |||
| Vitamin D(3) 24-hydroxylase (CYP24A1) | DME Info | Homo sapiens | [1], [5] | |||
| Calcidiol 1-monooxygenase (CYP27B1) | DME Info | Homo sapiens | [27] | |||
| Cytochrome P450 2A6 (CYP2A6) | DME Info | Homo sapiens | [28], [29] | |||
| Cytochrome P450 2B6 (CYP2B6) | DME Info | Homo sapiens | [30], [29] | |||
| Cytochrome P450 2C9 (CYP2C9) | DME Info | Homo sapiens | [29] | |||
| Cytochrome P450 2E1 (CYP2E1) | DME Info | Homo sapiens | [29] | |||
| Cytochrome P450 2J2 (CYP2J2) | DME Info | Homo sapiens | [3] | |||
| Cytochrome P450 2S1 (CYP2S1) | DME Info | Homo sapiens | [5] | |||
| Cytochrome P450 3A4 (CYP3A4) | DME Info | Homo sapiens | [31] | |||
| Dicarbonyl/L-xylulose reductase (DCXR) | DME Info | Homo sapiens | [32] | |||
| Dihydrofolate reductase (DHFR) | DME Info | Homo sapiens | [32] | |||
| Short-chain dehydrogenase/reductase retSDR1 (DHRS3) | DME Info | Homo sapiens | [3], [4] | |||
| Thymidylate kinase (DTYMK) | DME Info | Homo sapiens | [24] | |||
| Fatty acid desaturase 1 (FADS1) | DME Info | Homo sapiens | [1] | |||
| Gamma-Glu-X carboxypeptidase (GGH) | DME Info | Homo sapiens | [3] | |||
| L-glutamine amidohydrolase (GLS) | DME Info | Homo sapiens | [33] | |||
| Glutamine synthetase (GLUL) | DME Info | Homo sapiens | [32] | |||
| Histamine N-methyltransferase (HNMT) | DME Info | Homo sapiens | [3], [17] | |||
| Heparanase (HPSE) | DME Info | Homo sapiens | [34] | |||
| Estradiol 17-beta-dehydrogenase 1 (HSD17B1) | DME Info | Homo sapiens | [25] | |||
| Dihydrotestosterone oxidoreductase (HSD3B1) | DME Info | Homo sapiens | [25] | |||
| Methionine-tRNA ligase mitochondrial (MARS2) | DME Info | Homo sapiens | [4] | |||
| S-adenosylmethionine synthase 2 (MAT2A) | DME Info | Homo sapiens | [4], [32] | |||
| Microsomal glutathione S-transferase 2 (MGST2) | DME Info | Homo sapiens | [32] | |||
| Cysteinyl-conjugate N-acetyltransferase (NAT8) | DME Info | Homo sapiens | [1] | |||
| Nitric oxide synthase inducible (NOS2) | DME Info | Homo sapiens | [35] | |||
| Nitric oxide synthase endothelial (NOS3) | DME Info | Homo sapiens | [36] | |||
| Phosphodiesterase 7B (PDE7B) | DME Info | Homo sapiens | [18] | |||
| Phosphoribosylformylglycinamidine synthase (PFAS) | DME Info | Homo sapiens | [4] | |||
| Choline phosphatase 1 (PLD1) | DME Info | Homo sapiens | [3] | |||
| Purine nucleoside phosphorylase (PNP) | DME Info | Homo sapiens | [20] | |||
| Prostaglandin G/H synthase 2 (COX-2) | DME Info | Homo sapiens | [37] | |||
| Transglutaminase H (TGM2) | DME Info | Homo sapiens | [3], [2] | |||
| Thioredoxin reductase TR1 (TXNRD1) | DME Info | Homo sapiens | [38] | |||
| Thymidylate synthase (TYMS) | DME Info | Homo sapiens | [32] | |||
| UDP-glucuronosyltransferase 2B15 (UGT2B15) | DME Info | Homo sapiens | [39] | |||
| UDP-glucuronosyltransferase 2B17 (UGT2B17) | DME Info | Homo sapiens | [3] | |||
| Uridine phosphorylase 1 (UPP1) | DME Info | Homo sapiens | [1] | |||
| Xenobiotics-DME Activity Data | ||||||
| Xenobiotics-DME Activity Data | Cytochrome P450 1B1 (CYP1B1) | DME Info | IC50 > 50 microM | [11] | ||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

