| Synonyms |
Capval; Coscopin; Coscopin (VAN); Coscotabs; D01036; DB06174; DSSTox_CID_3385; DSSTox_GSID_23385; DSSTox_RID_77007; DTXSID4023385; EINECS 204-899-2; GTPL10212; Gnoscopine; H2069; HMS1569B08; HMS2096B08; HMS2269P05; HSDB 3372; HY-13716; Hederix (free base); Key-tusscapine; L-.alpha.-Narcotine; L-alpha-2-Methyl-8-methoxy-6,7-methylenedioxy-1-(6,7-dimethoxy-3-phthalidyl)-1,2,3,4-tetrahydroisoquinaline; L-alpha-Narcotine; L-alpha-Noscapine; Longactin; Longatin; Lopac0_000840; Lyobex; MCULE-5334942206; MLS000069475; MLS001060855; Methoxyhydrastine; NARCOTINE; NCGC00016388-01; NCGC00023230-02; NCGC00023230-04; NCGC00023230-05; NCGC00023230-07; NCGC00023230-08; NCGC00023230-10; NCI60_004322; NSC 5366; NSC 96350; NSC-121869; NSC-5366; NSC121869; NSC5366; Narcompren; Narcosine; Narcotin; Narcotine (8CI); Narcotine alkaloid; Narcotine, (+-)-; Narcotussin; Narkotin; Nectadon; Nicolane; Nipaxon; Noscapal; Noscapalin; Noscapin; Noscapina; Noscapina [INN-Spanish]; Noscapine (JP15/USP/INN); Noscapine (JP17/USP/INN); Noscapine (TN); Noscapine 1.0 mg/ml in Acetonitrile; Noscapine [USP:INN:BAN:JAN]; Noscapine dl-form; Noscapine, European Pharmacopoeia (EP) Reference Standard; Noscapine, United States Pharmacopeia (USP) Reference Standard; Noscapinum; Noscapinum [INN-Latin]; Noscopine; Noskapin; (+-)-Noscapine; (+-)-alpha-Narcotine; (-)-.alpha.-Narcotine; (-)-.alpha.-Norcotine; 8-Methoxyhydrastin; 8V32U4AOQU; A805851; AC-20272; AKNNEGZIBPJZJG-MSOLQXFVSA-N; AKOS000278036; API0003636; ARONIS24067; BBC/205; BBL012344; BDBM50424716; BIM-0048054.P001; BPBio1_000382; BRD-K89237706-001-03-8; BRN 0099933; BSPBio_000346; C-21952; C09592; CAS-128-62-1; CBMicro_048259; CC-33083; CCG-204096; CCRIS 9304; CHEBI:73237; CHEMBL364713; CS-5115
|
| Cross-matching ID |
- PubChem CID
- 275196
- PubChem SID
-
11783
; 71491
; 415752
; 605271
; 855860
; 7848099
; 7980174
; 8065369
; 9413073
; 10321868
; 11113349
; 11193550
; 11408713
; 11466591
; 11467711
; 11486301
; 14831210
; 14904386
; 17439397
; 24862408
; 41295460
; 47216689
; 47216690
; 47365103
; 48184909
; 48184910
; 48416344
; 49645498
; 49698554
; 50104123
; 50104124
; 50104125
; 53788781
; 57400976
; 57705206
; 61055762
; 75471156
; 85787957
; 87544097
; 87982965
; 90341457
; 92125312
; 92718085
; 103462008
; 103914348
; 104826459
; 113483048
; 115354111
; 118055360
; 121362983
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y8AW
- Formula
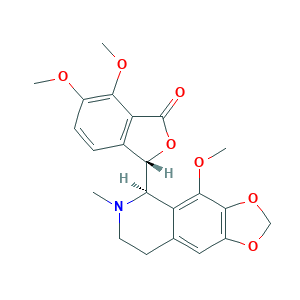
- C22H23NO7
- Canonical SMILES
- CN1CCC2=CC3=C(C(=C2C1C4C5=C(C(=C(C=C5)OC)OC)C(=O)O4)OC)OCO3
- InChI
- 1S/C22H23NO7/c1-23-8-7-11-9-14-20(29-10-28-14)21(27-4)15(11)17(23)18-12-5-6-13(25-2)19(26-3)16(12)22(24)30-18/h5-6,9,17-18H,7-8,10H2,1-4H3/t17-,18+/m1/s1
- InChIKey
- AKNNEGZIBPJZJG-MSOLQXFVSA-N
|