| Synonyms |
Cannabidiol; delta1(2)-trans-Cannabidiol; Epidiolex; QHMBSVQNZZTUGM-ZWKOTPCHSA-N; (-)-CBD; (-)-Cannabidiol; (-)-trans-2-p-Mentha-1,8-dien-3-yl-5-pentylresorcinol; (-)-trans-Cannabidiol; 1,3-Benzenediol, 2-(3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl)-5-pentyl-, (1R-trans)-; 13956-29-1; 19GBJ60SN5; 2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)-5-pentyl-benzene-1,3-diol; 2-[(1R,6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol; CBD; CHEBI:69478; CHEMBL190461; GWP42003-P; UNII-19GBJ60SN5
|
| Cross-matching ID |
- PubChem CID
- 644019
- PubChem SID
-
841235
; 8707245
; 12012585
; 14825860
; 14899058
; 24892861
; 43765781
; 57309735
; 57408178
; 57654032
; 80649036
; 103460177
; 104014242
; 104152845
; 109827355
; 113635231
; 134990571
; 135697550
; 136213730
; 142472550
; 160711726
; 162224688
; 178100971
; 198983043
; 224092533
; 226491388
; 242059005
; 250138861
; 252159978
; 252415453
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O1UZ
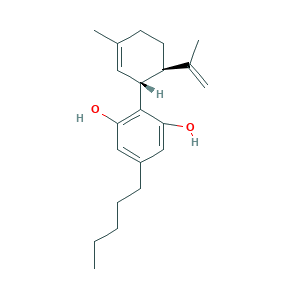
- Formula
- C21H30O2
- Canonical SMILES
- CCCCCC1=CC(=C(C(=C1)O)C2C=C(CCC2C(=C)C)C)O
- InChI
- 1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1
- InChIKey
- QHMBSVQNZZTUGM-ZWKOTPCHSA-N
|