Detail Information of Xenobiotics
| General Information of Xenobiotics (ID: XEO00538) | ||||||
|---|---|---|---|---|---|---|
| Xenobiotics Name |
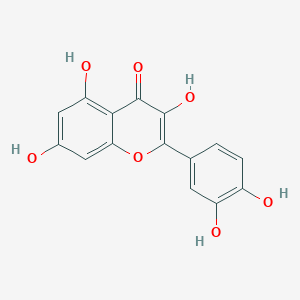
Quercetin
|
|||||
| Xenobiotics Type |
Pharmaceutical Agent(s)
|
|||||
| Classification |
Drug in Phase 1 Clinical Trial
|
|||||
| Structure |
<iframe style="width: 300px; height: 300px;" frameborder="0" src="https://embed.molview.org/v1/?mode=balls&cid=5280343"></iframe>
|
 |
||||
| 3D MOL | 2D MOL | |||||
| PubChem CID | ||||||
| DME(s) Modulated by This Xenobiotics | ||||||
| DME(s) Inhibited by This Xenobiotics | ||||||
| Alcohol dehydrogenase class-III (ADH5) | DME Info | Homo sapiens | [1] | |||
| Alcohol dehydrogenase class-V (ADH6) | DME Info | Homo sapiens | [2] | |||
| Aldo-keto reductase 1C2 (AKR1C2) | DME Info | Homo sapiens | [3] | |||
| Aldehyde dehydrogenase 5 (ALDHX) | DME Info | Homo sapiens | [3] | |||
| Aldehyde dehydrogenase 2 (ALDH2) | DME Info | Homo sapiens | [4] | |||
| Azoreductase (azoR) | DME Info | Escherichia coli | [5] | |||
| L,D-carboxypeptidase A (ldcA) | DME Info | Escherichia coli | [5] | |||
| Cytosolic branched aminotransferase (BCAT1) | DME Info | Homo sapiens | [1] | |||
| Beta-lactamase (blaB) | DME Info | Escherichia coli | [5] | |||
| Catalase (CAT) | DME Info | Homo sapiens | [6] | |||
| Chloramphenicolase (chlR) | DME Info | Escherichia coli | [5] | |||
| Glycoside hydrolase (cscA) | DME Info | Escherichia coli | [5] | |||
| NADPH-dependent curcumin reductase (curA) | DME Info | Escherichia coli | [5] | |||
| Microsomal cytochrome MCB5 (CYB5A) | DME Info | Homo sapiens | [7] | |||
| Aromatase (CYP19A1) | DME Info | Homo sapiens | [8] | |||
| Cytochrome P450 1A1 (CYP1A1) | DME Info | Homo sapiens | [9], [10] | |||
| Cytochrome P450 1A2 (CYP1A2) | DME Info | Homo sapiens | [11] | |||
| Cytochrome P450 1B1 (CYP1B1) | DME Info | Homo sapiens | [9], [10] | |||
| Vitamin D(3) 24-hydroxylase (CYP24A1) | DME Info | Homo sapiens | [12] | |||
| Mephenytoin 4-hydroxylase (CYP2C19) | DME Info | Homo sapiens | [11] | |||
| Cytochrome P450 2C8 (CYP2C8) | DME Info | Homo sapiens | [13] | |||
| Cytochrome P450 2C9 (CYP2C9) | DME Info | Homo sapiens | [11] | |||
| Cytochrome P450 2D6 (CYP2D6) | DME Info | Homo sapiens | [11] | |||
| Cytochrome P450 3A4 (CYP3A4) | DME Info | Homo sapiens | [11] | |||
| DOPA decarboxylase (DDC) | DME Info | Homo sapiens | [1] | |||
| Delta(24)-sterol reductase (DHCR24) | DME Info | Homo sapiens | [7] | |||
| Unclear metabolic mechanism (DME-unclear) | DME Info | Escherichia coli | [5] | |||
| Glutamate decarboxylase (gadB) | DME Info | Escherichia coli | [5] | |||
| Glutathione S-transferase alpha-1 (GSTA1) | DME Info | Homo sapiens | [14] | |||
| Glutathione S-transferase pi (GSTP1) | DME Info | Homo sapiens | [15] | |||
| HMG-CoA reductase (HMGCR) | DME Info | Homo sapiens | [3] | |||
| Histamine N-methyltransferase (HNMT) | DME Info | Homo sapiens | [1] | |||
| Heparanase (HPSE) | DME Info | Homo sapiens | [16] | |||
| Estradiol 17-beta-dehydrogenase 2 (HSD17B2) | DME Info | Homo sapiens | [17] | |||
| Peroxisomal multifunctional enzyme 2 (HSD17B4) | DME Info | Homo sapiens | [3] | |||
| D-Lactate dehydrogenase (ldhA) | DME Info | Escherichia coli | [5] | |||
| D-Lactate dehydrogenase (ldhA) | DME Info | Pseudomonas aeruginosa | [18] | |||
| Methionine-tRNA ligase mitochondrial (MARS2) | DME Info | Homo sapiens | [1] | |||
| S-adenosylmethionine synthase 1 (MAT1A) | DME Info | Homo sapiens | [1] | |||
| Microsomal glutathione S-transferase 2 (MGST2) | DME Info | Homo sapiens | [6] | |||
| Molybdopterin-dependent enzyme (molD) | DME Info | Escherichia coli | [5] | |||
| Glutamate racemase (MurI) | DME Info | Escherichia coli | [5] | |||
| Methylarsonite methyltransferase N6AMT1 (N6AMT1) | DME Info | Homo sapiens | [7] | |||
| Arylamine N-acetyltransferase (NAT) | DME Info | Pseudomonas aeruginosa | [18] | |||
| N-ethylmaleimide reductase (nemA) | DME Info | Escherichia coli | [5] | |||
| Oxygen-insensitive NADPH nitroreductase A (nfsA) | DME Info | Escherichia coli | [5] | |||
| Oxygen-insensitive NADPH nitroreductase B (nfsB) | DME Info | Escherichia coli | [5] | |||
| Nitric oxide synthase inducible (NOS2) | DME Info | Homo sapiens | [1] | |||
| Ecto-5'-nucleotidase (NT5E) | DME Info | Homo sapiens | [19] | |||
| NADH dehydrogenase (nuoE) | DME Info | Streptomyces griseus | [5] | |||
| Phosphoglucomutase 1 (PGM1) | DME Info | Homo sapiens | [1] | |||
| Choline phosphatase 1 (PLD1) | DME Info | Homo sapiens | [20] | |||
| Hydroxybenzoate 3-monooxygenase (pobA) | DME Info | Pseudomonas aeruginosa | [18] | |||
| NADPH-cytochrome P450 reductase (CPR) | DME Info | Homo sapiens | [4] | |||
| Prostaglandin G/H synthase 1 (COX-1) | DME Info | Homo sapiens | [21] | |||
| Prostaglandin G/H synthase 2 (COX-2) | DME Info | Homo sapiens | [22] | |||
| Xylosyl phosphatase (PXYLP1) | DME Info | Homo sapiens | [3] | |||
| Transglutaminase H (TGM2) | DME Info | Homo sapiens | [1] | |||
| Thymidylate synthase (TYMS) | DME Info | Homo sapiens | [1] | |||
| Tyramine oxidase (tynA) | DME Info | Escherichia coli | [5] | |||
| Beta-glucuronidase (uidA) | DME Info | Escherichia coli | [5] | |||
| DME(s) Induced by This Xenobiotics | ||||||
| Adenosine aminohydrolase (ADA) | DME Info | Homo sapiens | [23] | |||
| Arylsulfatase A (ARSA) | DME Info | Homo sapiens | [24] | |||
| Carbonic anhydrase II (CA2) | DME Info | Homo sapiens | [25] | |||
| Cytidine aminohydrolase (CDA) | DME Info | Homo sapiens | [15] | |||
| Carboxylesterase 2 (CES2) | DME Info | Homo sapiens | [26] | |||
| Calcidiol 1-monooxygenase (CYP27B1) | DME Info | Homo sapiens | [27] | |||
| Cytochrome P450 2A6 (CYP2A6) | DME Info | Homo sapiens | [28] | |||
| Sodium/potassium-transporting ATPase gamma (FXYD2) | DME Info | Homo sapiens | [24] | |||
| N-acetyl-beta-glucosaminidase beta (HEXB) | DME Info | Homo sapiens | [29], [30] | |||
| Metallothionein-2A (MT2A) | DME Info | Homo sapiens | [30] | |||
| Quinone reductase 1 (NQO1) | DME Info | Homo sapiens | [29] | |||
| Superoxide dismutase 1 (SOD1) | DME Info | Homo sapiens | [30] | |||
| Thioredoxin reductase TR1 (TXNRD1) | DME Info | Homo sapiens | [24] | |||
| UDP-glucuronosyltransferase 1A1 (UGT1A1) | DME Info | Homo sapiens | [31] | |||
| UDP-glucuronosyltransferase 1A6 (UGT1A6) | DME Info | Homo sapiens | [32] | |||
| Xanthine dehydrogenase/oxidase (XDH) | DME Info | Homo sapiens | [33] | |||
| Xenobiotics-DME Activity Data | ||||||
| Xenobiotics-DME Activity Data | Aromatase (CYP19A1) | DME Info | IC50 = 0.012 microM | [8] | ||
| Cytochrome P450 1A1 (CYP1A1) | DME Info | IC50 = 1.191 microM | [9], [10] | |||
| Cytochrome P450 1A2 (CYP1A2) | DME Info | IC50 = 1.55 microM | [11] | |||
| Cytochrome P450 1B1 (CYP1B1) | DME Info | IC50 = 0.077 microM | [9], [10] | |||
| Mephenytoin 4-hydroxylase (CYP2C19) | DME Info | IC50 = 10.5 microM | [11] | |||
| Cytochrome P450 2C8 (CYP2C8) | DME Info | IC50 = 1.2 microM | [13] | |||
| Cytochrome P450 2C9 (CYP2C9) | DME Info | IC50 = 10.2 microM | [11] | |||
| Cytochrome P450 2D6 (CYP2D6) | DME Info | IC50 = 18.8 microM | [11] | |||
| Cytochrome P450 3A4 (CYP3A4) | DME Info | IC50 = 2.11 microM | [11] | |||
| Estradiol 17-beta-dehydrogenase 2 (HSD17B2) | DME Info | IC50 = 1.54 microM | [17] | |||
| Prostaglandin G/H synthase 2 (COX-2) | DME Info | IC50 = 28.6 microM | [22] | |||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

