Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1572) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Thalidomide
|
|||||
| Synonyms |
Talargan; Talismol; Telargean; Tensival; Thalinette; Thalomid; Theophilcholine; Valgraine; thalidomide; (+/-)-THALIDOMIDE; Algosediv; Asmadion; Calmorex; Contergan; Corronarobetin; Ectiluran; Enterosediv; Gastrinide; Glutanon; Hippuzon; Neosedyn; Neosydyn; Nerosedyn; Neufatin; Neurodyn; Neurosedin; Neurosedym; Nevrodyn; Noctosediv; Pantosediv; Polygripan; Profarmil; Psycholiquid; Psychotablets; Quetimid; Quietoplex; Sandormin; Sedimide; Sedisperil; Sedoval; Shinnibrol; Softenil; Softenon; 2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione; 50-35-1
|
|||||
| Indication | Breast cancer [ICD11: 2C60] | Approved | [1] | |||
| Structure |
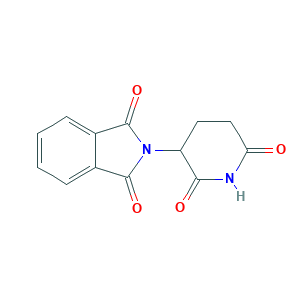 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 258.23 | Topological Polar Surface Area | 83.6 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Thalidomide was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | |||||
| 3 | Thalidomide metabolism and hydrolysis: mechanisms and implications | |||||
| 4 | Metabolism of thalidomide in human microsomes, cloned human cytochrome P-450 isozymes, and Hansen's disease patients. J Biochem Mol Toxicol. 2000;14(3):140-7. | |||||
| 5 | Thalidomide metabolism by the CYP2C subfamily. Clin Cancer Res. 2002 Jun;8(6):1964-73. | |||||
| 6 | Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formation of a glutathione conjugate. Chem Res Toxicol. 2010 Jun 21;23(6):1018-24. | |||||
| 7 | Pharmacogenetic associations of CYP2C19 genotype with in vivo metabolisms and pharmacological effects of thalidomide. Cancer Biol Ther. 2002 Nov-Dec;1(6):669-73. | |||||
| 8 | Chiral inversion and hydrolysis of thalidomide: mechanisms and catalysis by bases and serum albumin, and chiral stability of teratogenic metabolites | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

